Abstract
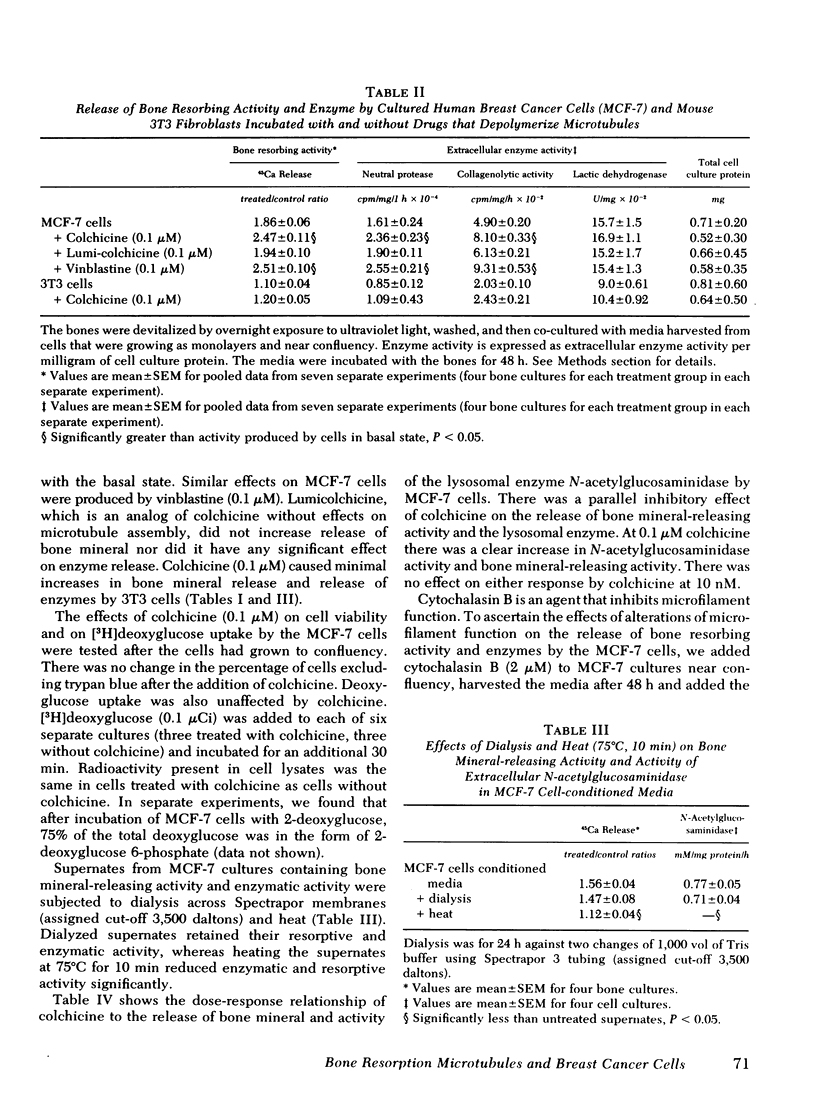
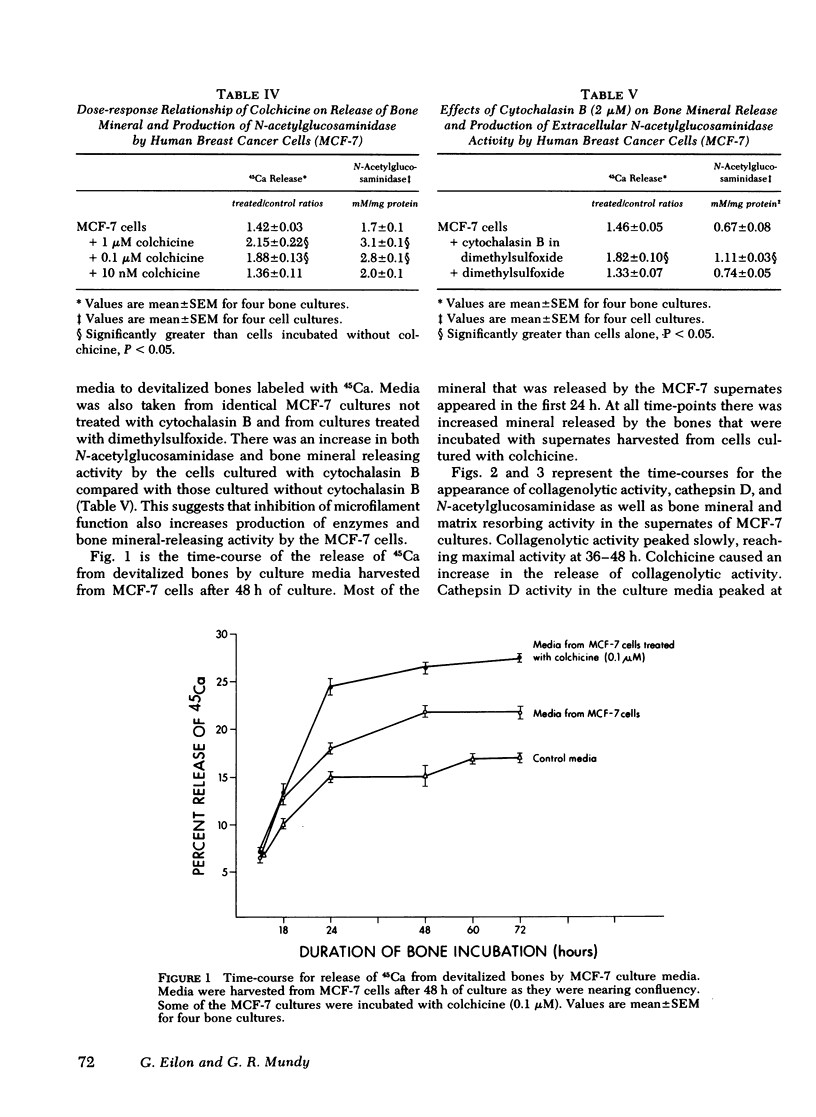
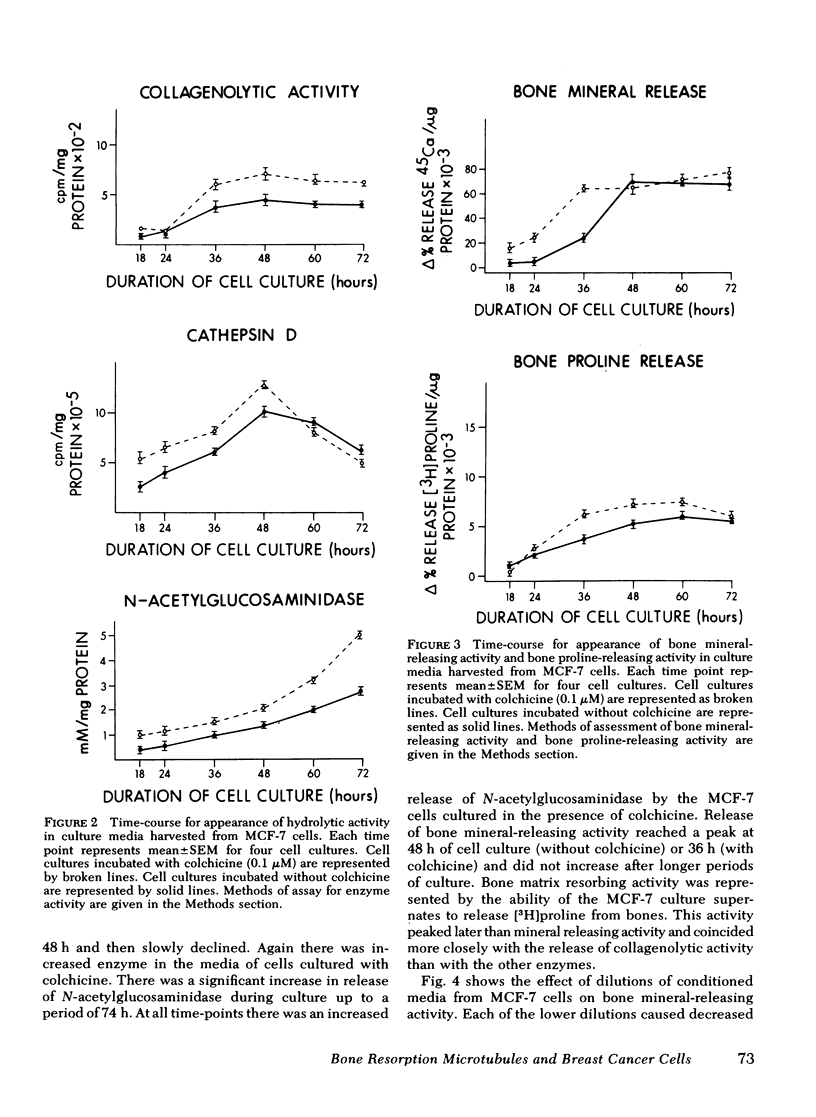
When supernates from the established human breast cancer cell line MCF-7 were applied to fetal rat long bones that had been labeled with 45Ca and devitalized to remove endogenous bone cells, mineral was released from the bones. The release of bone mineral by MCF-7 supernates was associated with increased basal release of hydrolytic enzyme activity by the tumor cells. The basal release of lysosomal enzymes and collagenolytic activity by MCF-7 cells with approximately twice that of mouse 3T3 cells, which did not cause mineral release by the fetal rat bones. Release of hydrolytic enzymes and bone mineral-releasing activity was increased by colchicine and vinblastine, drugs that inhibit microtubule assembly, but not affected by lumicolchicine. Time-course experiments performed on MCF-7 cells with or without colchicine showed that release of cathepsin D and collagenolytic activity was associated more closely with release of bone mineral and degradation of bone matrix than was the release of N-acetylglucosaminidase. The release of previously incorporated [3H]proline from the bones exposed to MCF-7 cell cultures was more closely associated with release of collagenolytic activity by MCF-7 cells than with release of cathepsin D or N-acetylglucosaminidase. These data suggest that breast cancer-mediated bone resorption in vitro is positively correlated with release of hydrolytic enzymes by the tumor cells, and release of these enzymes is enhanced by disassembly of microtubules.
Full text
PDF

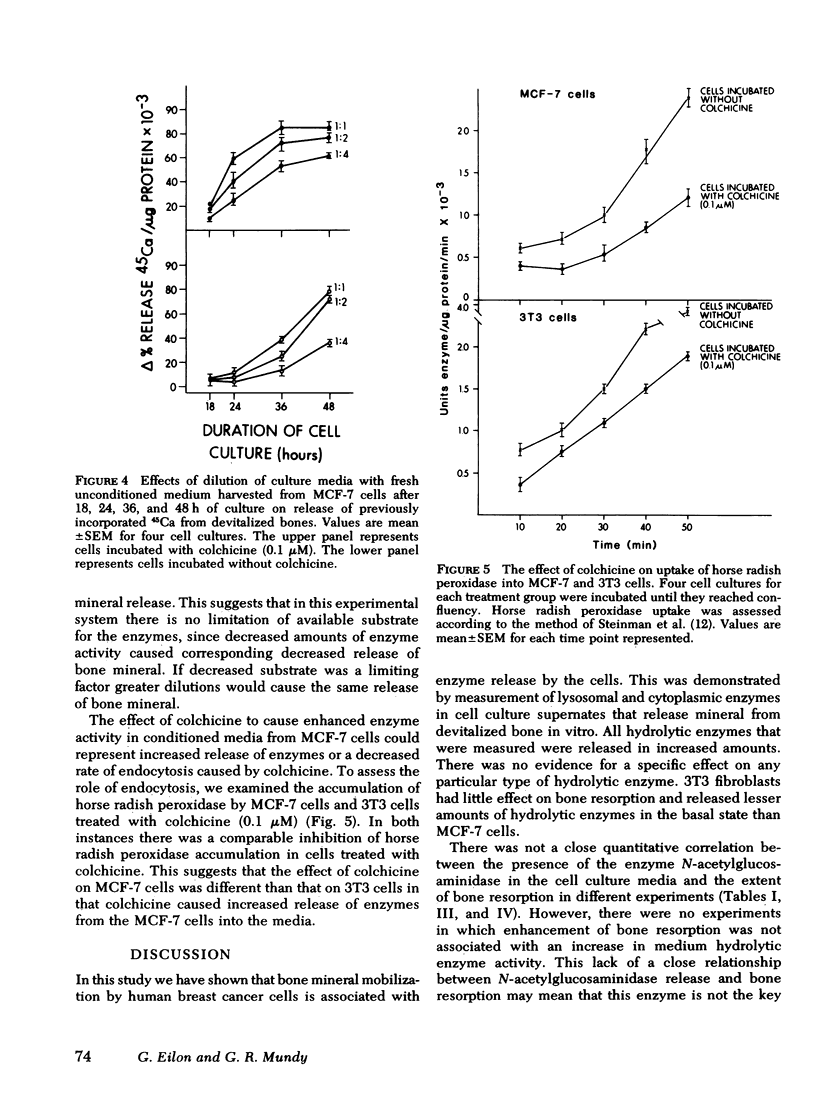


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGERS J. D., GWATKIN R. B., HEYNER S. Growth of embryonic avian and mammalian tibiae on a relatively simple chemically defined medium. Exp Cell Res. 1961 Oct;25:41–58. doi: 10.1016/0014-4827(61)90305-6. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Analogous ultrastructure and surface properties during capping and phagocytosis in leukocytes. J Cell Biol. 1978 Jun;77(3):789–804. doi: 10.1083/jcb.77.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978 Dec 14;276(5689):726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- Eilon G., Raisz L. G. Comparison of the effects of stimulators and inhibitors of resorption on the release of lysosomal enzymes and radioactive calcium from fetal bone in organ culture. Endocrinology. 1978 Dec;103(6):1969–1975. doi: 10.1210/endo-103-6-1969. [DOI] [PubMed] [Google Scholar]
- Galasko C. S., Burn J. I. Hypercalcaemia in patients with advanced mammary cancer. Br Med J. 1971 Sep 4;3(5774):573–577. doi: 10.1136/bmj.3.5774.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtrop M. E., Raisz L. G., Simmons H. A. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol. 1974 Feb;60(2):346–355. doi: 10.1083/jcb.60.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malawista S. E. Colchicine: a common mechanism for its anti-inflammatory and anti-mitotic effects. Arthritis Rheum. 1968 Apr;11(2):191–197. doi: 10.1002/art.1780110210. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES W. J., Jr, FIMOGNARI G. M. AN IMPROVED PROCEDURE FOR THE PREPARATION OF CRYSTALLINE LACTIC DEHYDROGENASE FROM HOG HEART. J Biol Chem. 1963 Dec;238:3853–3858. [PubMed] [Google Scholar]
- Shevach E. M., Herberman R., Frank M. M., Green I. Receptors for complement and immunoglobulin on human leukemic cells and human lymphoblastoid cell lines. J Clin Invest. 1972 Aug;51(8):1933–1938. doi: 10.1172/JCI106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J Cell Biol. 1973 Jul;58(1):27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Kresse H., Meinhard U., Holtfrerich D. Studies on secretion and endocytosis of macromolecules by cultivated skin fibroblasts. Effects of anti-microtubular agents on secretion and endocytosis of lysosomal hydrolases and of sulphated glycosaminoglycans. Biochem J. 1978 Feb 15;170(2):313–320. doi: 10.1042/bj1700313. [DOI] [PMC free article] [PubMed] [Google Scholar]


