Abstract
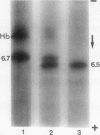
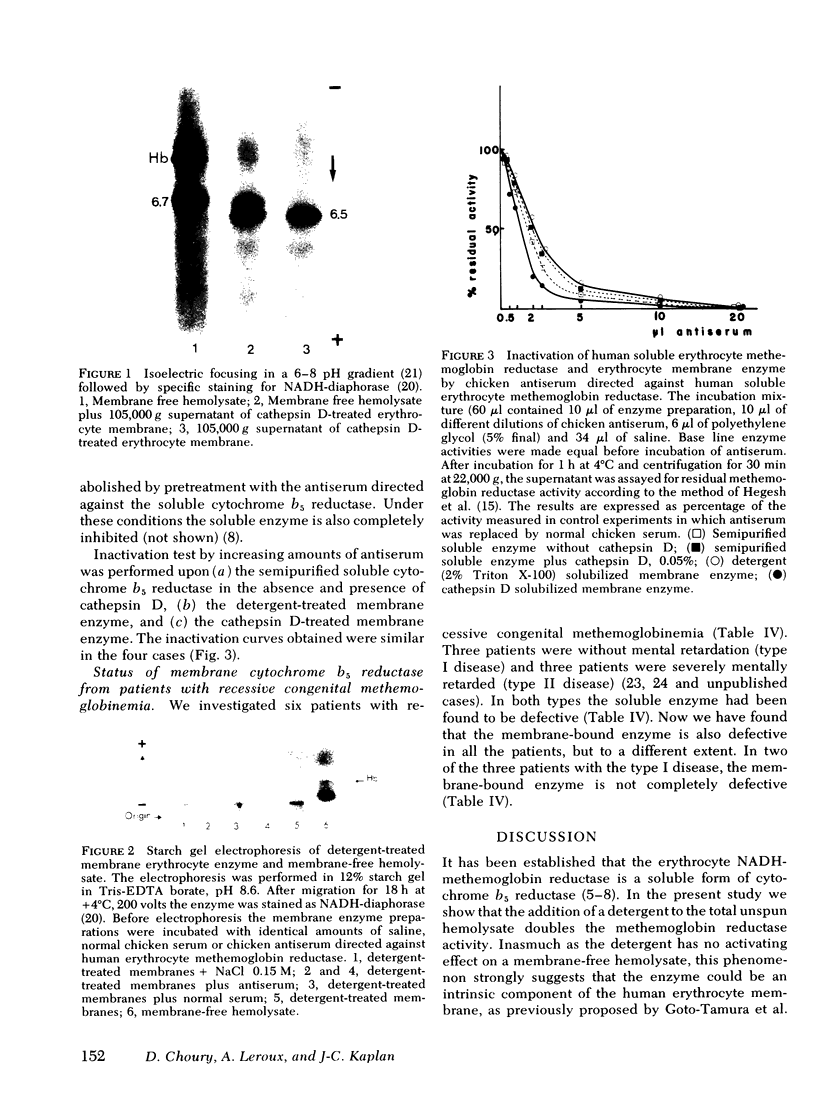
In this study we present evidence that in human erythrocytes NADH-cytochrome b5 reductase (methemoglobin reductase) is not only soluble but also tightly bound to the membrane. The membrane methemoglobin reductase-like activity is unmasked by Triton X-100 treatment, and represents about half of the total activity in the erythrocytes. Like the amphiphilic microsomal-bound cytochrome b5 reductase, the erythrocyte membrane-bound enzyme is solubilized by cathepsin D. Because this treatment is effective on unsealed ghosts but not on resealed (inside-in) ghosts, it is concluded that the enzyme is strongly bound to the inner face of the membrane. The erythrocyte membrane enzyme is antigenically similar to the soluble enzyme. The two forms of enzyme are specified by the same gene, in that both were found defective in six patients with recessive congenital methemoglobinemia. We suggest that the cytochrome b5 reductase of the erythrocyte membrane is the primary gene product. A posttranslational partial proteolysis probably gives rise to the soluble form of the enzyme, which serves as a methemoglobin reductase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Blume K. G., Kaplan J. C., Löhr G. W., Ramot B., Valentine W. N. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977 Feb;35(2):331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Board P. G., Agar N. S., Gruca M., Shine R. Methaemoglobin and its reduction in nucleated erythrocytes from reptiles and birds. Comp Biochem Physiol B. 1977;57(3):265–267. doi: 10.1016/0305-0491(77)90155-9. [DOI] [PubMed] [Google Scholar]
- Choury D., Kaplan J. C. Diaphorase P: a new fetal isozyme identified in human placenta. Biochim Biophys Acta. 1980;613(1):18–25. doi: 10.1016/0005-2744(80)90187-4. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Enomoto K. I., Sato R. Asymmetric binding of cytochrome b5 to the membrane of human erythrocyte ghosts. Biochim Biophys Acta. 1977 Apr 1;466(1):136–147. doi: 10.1016/0005-2736(77)90214-0. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Povey S., Bobrow M., Solomon E., Boyd Y., Carritt B. Assignment of the DIA1 locus to chromosome 22. Ann Hum Genet. 1977 Oct;41(2):151–155. doi: 10.1111/j.1469-1809.1977.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Gacon G., Lostanlen D., Labie D., Kaplan J. C. Interaction between cytochrome b5 and hemoglobin: involvement of beta 66 (E10) and beta 95 (FG2) lysyl residues of hemoglobin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1917–1921. doi: 10.1073/pnas.77.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg H., Crane F. L., Morré D. J. NADH oxidoreductase of mouse liver plasma membranes. J Biol Chem. 1979 Apr 10;254(7):2491–2498. [PubMed] [Google Scholar]
- Goto-Tamura R., Takesue Y., Takesue S. Immunological similarity between NADH-cytochrome b5 reductase of erythrocytes and liver microsomes. Biochim Biophys Acta. 1976 Feb 16;423(2):293–302. doi: 10.1016/0005-2728(76)90186-9. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968 Aug;72(2):339–344. [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Slaughter S. R., Douglas R. H., Sannes L. J., Sahagian G. G. Erythrocyte cytochrome b5; structure, role in methemoglobin reduction, and solubilization from endoplasmic reticulum. Prog Clin Biol Res. 1978;21:199–216. [PubMed] [Google Scholar]
- Ito A. Evidence obtained by cathepsin digestion of microsomes for the assembly of cytochrome b5 and its reductase in the membrane. J Biochem. 1974 Apr;75(4):787–793. doi: 10.1093/oxfordjournals.jbchem.a130451. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato R. Purification by means of detergents and properties of cytochrome b5 from liver microsomes. J Biol Chem. 1968 Sep 25;243(18):4922–4923. [PubMed] [Google Scholar]
- Junien C., Vibert M., Weil D., Van-Cong N., Kaplan J. C. Assignment of NADH-cytochrome b5 reductase (DIA1 locus) to human chromosome 22. Hum Genet. 1978 Jun 27;42(3):233–239. doi: 10.1007/BF00291301. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoresis of red cell NADH- and NADPH-diaphorases in normal subjects and patients with congenital methemoglobinemia. Biochem Biophys Res Commun. 1967 Nov 30;29(4):605–610. doi: 10.1016/0006-291x(67)90529-3. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Leroux A., Bakouri S., Grangaud J. P., Benabadji M. La lésion enzymatique dans la méthémoglobinémie congénitale récessive avec encéphalopathie. Description d'une nouvelle variante déficitaire de NADH-diaphorase (variante Beni-Messous) Nouv Rev Fr Hematol. 1974 Nov-Dec;14(6):755–770. [PubMed] [Google Scholar]
- Kaplan J. C., Leroux A., Beauvais P. Formes cliniques et biologiques du déficit en cytochrome b5 réductase. C R Seances Soc Biol Fil. 1979;173(2):368–379. [PubMed] [Google Scholar]
- Kuma F., Prough R. A., Masters B. S. Studies on methemoglobin reductase. Immunochemical similarity of soluble methemoglobin reductase and cytochrome b5 of human erythrocytes with NADH-cytochrome b5 reductase and cytochrome b5 of rat liver microsomes. Arch Biochem Biophys. 1976 Feb;172(2):600–607. doi: 10.1016/0003-9861(76)90113-2. [DOI] [PubMed] [Google Scholar]
- Kuwahara S., Okada Y., Omura T. Evidence for molecular identity of microsomal and mitochondrial NADH-cytochrome b5 reductases of rat liver. J Biochem. 1978 Apr;83(4):1049–1059. doi: 10.1093/oxfordjournals.jbchem.a131993. [DOI] [PubMed] [Google Scholar]
- Leroux A., Torlinski L., Kaplan J. C. Soluble and microsomal forms of NADH-cytochrome beta 5 reductase from human placenta. Similarity with NADH-methemoglobin reductase from human erythrocytes. Biochim Biophys Acta. 1977 Mar 15;481(1):50–62. doi: 10.1016/0005-2744(77)90136-x. [DOI] [PubMed] [Google Scholar]
- Lostanlen D., Vieira de Barros A., Leroux A., Kaplan J. C. Soluble NADH-cytochrome b5 reductase from rabbit liver cytosol: partial purification and characterization. Biochim Biophys Acta. 1978 Sep 11;526(1):42–51. doi: 10.1016/0005-2744(78)90288-7. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Marchesi V. T., Tillack T. W. Physical and chemical properties of a protein isolated from red cell membranes. Biochemistry. 1970 Jan 6;9(1):50–57. doi: 10.1021/bi00803a007. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R. Purification and properties of the intact form of NADH-cytochrome b5 reductase from rabbit liver microsomes. J Biochem. 1975 Nov;78(5):1057–1073. doi: 10.1093/oxfordjournals.jbchem.a130983. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R., Sakakibara R., Wada H. Reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase: location of the hydrophobic, membrane-binding region at the carboxyl-terminal end and the masked amino terminus. Biochemistry. 1978 Jul 11;17(14):2839–2834. doi: 10.1021/bi00607a020. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., McGRAW J. C. Purification and properties of diphosphopyridine nucleotide diaphorase of human erythrocytes. J Biol Chem. 1962 Jan;237:249–252. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The purification and properties of microsomal cytochrome reductase. J Biol Chem. 1957 Oct;228(2):785–799. [PubMed] [Google Scholar]
- Sargent J. R., St Louis P. J., Blair P. A. Isolation of NADH-cytochrome b5 oxidoreductase from rat liver microsomes. Biochim Biophys Acta. 1970 Dec 8;223(2):339–348. doi: 10.1016/0005-2728(70)90190-8. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Takesue S., Omura T. Solubilization of NADH-cytochrome b5 reductase from liver microsomes by lysosomal digestion. J Biochem. 1970 Feb;67(2):259–266. doi: 10.1093/oxfordjournals.jbchem.a129249. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Canessa M. Nicotinamide-adenine dinucleotide dehydrogenase activity of human erythrocyte membranes. Biochim Biophys Acta. 1966 May 12;120(1):165–169. doi: 10.1016/0926-6585(66)90290-1. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Cellino M., Canessa-Fischer M. The relation between membrane structure and NADH: (acceptor) oxidoreductase activity of erythrocyte ghosts. Arch Biochem Biophys. 1969 Jan;129(1):336–345. doi: 10.1016/0003-9861(69)90184-2. [DOI] [PubMed] [Google Scholar]