Abstract
Yessotoxin is a marine phycotoxin that induces motor alterations in mice after intraperitoneal injection. In primary cortical neurons, yessotoxin treatment induced a caspase-independent cell death with an IC50 of 4.27 nM. This neurotoxicity was enhanced by 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid and partially blocked by amiloride. Unlike previous studies, yessotoxin did not increase cyclic adenosine monophosphate levels or produce any change in phosphodiesterase 4 steady state expression in triple transgenic neurons. Since phosphodiesterases (PDEs) are engaged in learning and memory, we studied the in vitro effect of the toxin against Alzheimer’s disease hallmarks and observed that pretreatment of cortical 3xTg-AD neurons with a low nanomolar concentration of yessotoxin showed a decrease expression of hyperphosphorylated tau isoforms and intracellular accumulation of amyloid-beta. These effects were accompanied with an increase in the level of the inactive isoform of the glycogen synthase kinase 3 and also by a translocation of protein kinase C from cytosol to membrane, pointing to its activation. In fact, inhibition of protein kinase C with GF109203X blocked the effect of yessotoxin over tau protein. The data presented here shows that 1 nM yessotoxin activates protein kinase C with beneficial effects over the main Alzheimer’s disease hallmarks, tau and Aβ, in a cellular model obtained from 3xTg-AD fetuses.
Keywords: Marine toxin, yessotoxin, Alzheimer’s disease, protein kinase C
Yessotoxin (YTX) is a marine phycotoxin with more than 40 analogues isolated for the first time in Japanese waters. It is produced by different dinoflagellates, among them, Protoceratium reticulatum and Lingulodinium polyedrum.1−3 Originally, the toxin was included with the okadaic acid (OA) in the group of diarrheic shellfish poisoning (DSP) toxins, because they used to appear together during toxic episodes. Later, it was separated in its own group, due to different biological origin and different in vivo effects.4
One of the main differences with OA is that YTX shows no inhibition of protein phosphatase 2A (PP2A).5 Recently, it has been observed that, in fact, YTX and OA show an immunoregulatory effect over lymphocytes, although through protein kinase C (PKC) mediated mechanisms in the case of YTX and through PP2A mechanisms in the case of OA.6
PKC is widely expressed in neurons, and it is implicated in neuroprotection, synaptic function, and plasticity. This turns it into an important molecule in learning and memory, and its signaling disruption causes impairment in these processes.7−9 Moreover, reduced PKC levels were found in samples from patients with Alzheimer’s disease (AD).10 Several pieces of evidence indicate that amyloid beta (Aβ) peptide can reduce PKC levels and also block the activation and normal function of the enzyme.11 All these findings indicate that PKC activators may constitute an interesting target for AD related pathology, as PKC isoforms are involved in memory processing and the enzyme can inhibit glycogen synthase 3 kinase through its activation.12 One example is that, in fact, bryostatin-1, an agonist of classic and novel PKC isoforms, reduces Aβ40 and Aβ42 in a double transgenic model of AD at subnanomolar concentrations, enhancing the secretion of the α-secretase soluble APP product.13
Initial reports on the mechanism of action of YTX described it as a phosphodiesterase (PDE) activator that decreased cyclic adenosine monophosphate (cAMP) levels in human lymphocytes.14 This effect was dependent on the presence of calcium in the extracellular medium. However, later reports described that PDE inhibition induced by YTX, measured by electrochemical and colorimetric methods,15 had no effect on cAMP levels in cardiomyocytes,16 pointing to different effects depending on cell type and bringing up doubts about the specific role of YTX in PDE signaling. In platelets, it has been shown that PKC was engaged in the activation of PDE3 as happens with other human cellular models,17,18 showing a link between these two enzymes. PDE modulators have been studied for their possible therapeutic effect in neurodegenerative diseases. For example, rolipram, a PDE4 inhibitor, has shown beneficial effects against Aβ induced memory and cognitive deficits19,20 through the increase of the intracellular cAMP levels available in the brain, hence activating protein kinase A (PKA) with a consequent down regulation of the cAMP response element binding (CREB) protein21,22 and an increase in the cAMP/CREB signaling in the brain.
It is well-known that YTX is also an apoptotic inducer in primary cultures and cell lines, with observed differences among cell types in concentrations and the protein pathways involved.23 This effect has made yessotoxin an interesting compound for cancer studies. Another type of programmed cell death, recently reported for YTX in the BC3H1 myoblast cell line, is paraptosis.24 This kind of programmed cellular death is typical of neurons overcoat in neurodegenerative diseases as Huntington’s disease and amyotrophic lateral sclerosis.25,26
So, in this work, we study the effects of YTX over primary cortical neurons for the first time. Taking advantage of the previous knowledge, we focused the study on YTX-mediated effects over PDE and PKC and the possibilities of this compound for the treatment of neurodegenerative diseases.
Results and Discussion
Yessotoxin-Induced Cytotoxicity
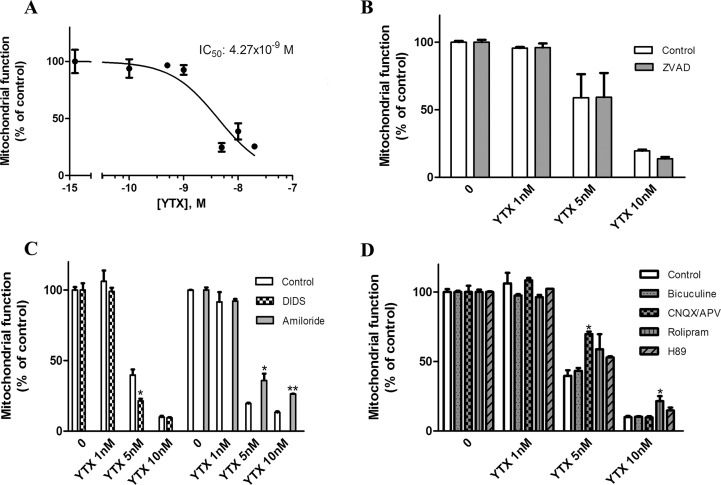
Although the nervous system was pointed as one target of YTX, with motor alterations in mice after intraperitoneal injection and histopathological damage in Purkinje cells,27 only the effects of YTX in cultured cerebellar neurons have been tested until now.28 As the cytotoxic effect of YTX in primary cortical neurons has not been evaluated yet, we tested the viability of these neurons obtained from Swiss mice after exposure to different YTX concentrations by the MTT assay. Concentrations ranging from 0.5 to 20 nM were added to the extracellular medium for 72 h. As shown in Figure 1A, YTX caused a concentration-dependent decrease in cellular viability. At 20 nM, YTX reduced cellular viability to 25.06 ± 0.49% with a half maximal inhibitory concentration (IC50) of 4.27 nM (95% confidence interval: 2.79–6.5 nM) (Figure 1A). Previous works demonstrated that YTX is an apoptotic inducer in different mammalians cells,23 but recently another kind of YTX induced death has been reported, paraptosis, a caspase independent cell death.24 So, in order to clarify if YTX-induced death in primary cortical neurons was apoptosis or another programmed cell death, three YTX concentration were chosen, 1, 5, and 10 nM, and a well-known cell-permeant caspase inhibitor, carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD) was added to the cellular medium for 48 h. As shown in Figure 1B, co-incubation with 100 μM Z-VAD did not modify YTX effects over neuronal viability, pointing to a mechanism independent of caspase activation.
Figure 1.
Effect of YTX on primary cortical neurons viability. (A) Dose-reponse curve indicating the effect of different YTX concentrations added to the culture medium during 72 h over neuronal viability measured by the MTT reduction assay. (B) Evaluation of caspase participation in YTX-induced toxicity. Addition of the caspase inhibitor Z-VAD 100 μM to the extracellular medium with 1, 5, and 10 nM YTX did not produce any effect over the YTX-induced cortical cell death. (C) Effect of anion channel modulators over YTX-induced toxicity. DIDS 500 μM or 10 μM amiloride was added to the extracellular medium with 1, 5, and 10 nM YTX. DIDS cotreatment increased the 5 nM YTX toxicity, whereas amiloride coincubation partially blocked YTX effects. (D) Evaluation of the participation of neurotransmitter receptors and kinase modulators in the YTX-induced death. The GABAergic inhibitor bicuculline 100 μM, glutamatergic modulators 200 and 100 μM CNQX and APV and the PDE4 and PKA inhibitor, 10 μM rolipram and 5 μM H89 were added to the cellular medium with 1, 5, and 10 nM YTX. Only the glutamatergic inhibitor mixture and the PDE4 inhibitor rolipram produced certain decrease in YTX induced neurotoxicity.
Effect of Anion Channel Modulators over YTX Neurotoxicity
As Pérez-Gómez and colleagues described for cerebellar neurons,28 granulation and weakening of neurites, followed by cytoplasmic vacuolation and cellular swelling, were observed in YTX treated primary neurons. Swollen cells suggest ionic alterations, so to further the cellular mechanism involved in YTX cytotoxicity, we studied the effect of several drugs implicated in anion homeostasis. Treatment of cortical neurons with the chloride channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) at a concentration of 500 μM did not affect cellular viability, but when the compound was incubated with YTX, the toxicity elicited by 5 nM YTX was 45.2 ± 9.4% (p = 0.041) higher than the toxicity elicited by the toxin alone. However, at 10 nM, with high neuronal damage, the percentage of dead neurons was almost the same. Meanwhile, cotreatment of cortical neurons with 10 μM of the Na+/H+ exchanger blocker amiloride and YTX showed that 5 nM YTX provides 183.9 ± 19.9% (p = 0.03) of mitochondrial activity versus neurons treated with YTX and this increase was maintained even at 10 nM YTX, in which case the percentage was 200.04 ± 10.4% (p = 0.007) versus 10 nM YTX alone (Figure 1C), showing a smaller toxic effect of YTX in the presence of amiloride.
Effect of Neurotransmitters and Enzyme Modulators over YTX-Induced Toxicity
We studied the effect of different neurotransmitters on YTX toxicity. For this purpose, two glutamate receptors antagonists, 2-amino-5-phosphonopentanoic acid (APV) and 7-nitro-2,3-dioxo-1,4-dihydroquinoxaline-6-carbonitrile (CNQX), 20 and 100 μM respectively, and 100 μM bicuculline, a γ-aminobutyric acid (GABA) receptor antagonist, were added to the extracellular medium with YTX. As can be seen in Figure 1D, the combination of the two glutamate receptor antagonists partially blocked the neurotoxicity elicited by YTX at 5 nM (p = 0.022), but failed at higher toxin concentrations, whereas bicuculline was ineffective at all the concentrations. Since YTX may act as a PDE activator, PDE4 inhibitor rolipram (10 μM) and the protein kinase A (PKA) inhibitor H89 (5 μM) were tested. As shown in Figure 1D, rolipram was able to partially inhibit the neuronal death elicited by 10 nM YTX (p = 0.017) while inhibition of PKA did not affect the decrease in cell viability produced by YTX.
Yessotoxin Effects in Phosphodiesterase 4 Expression and cAMP Release
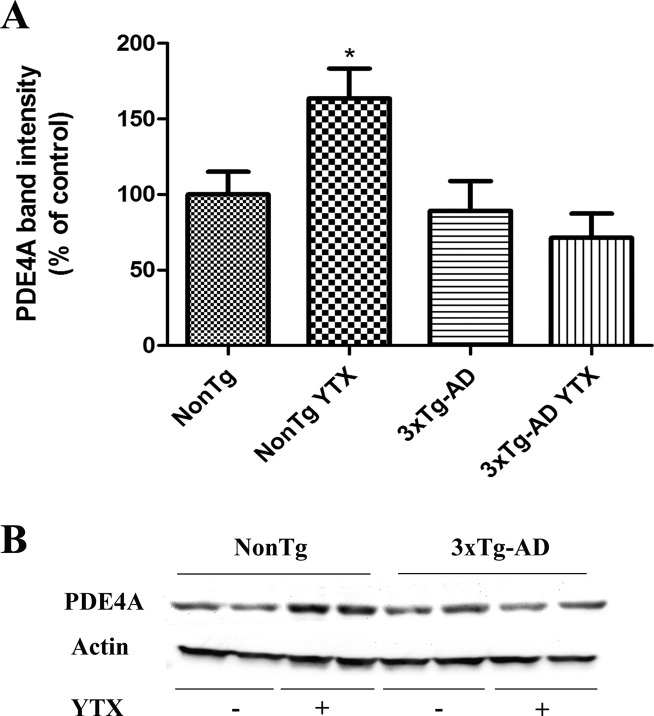
PDE4 has been shown to be engaged in memory processes,21 and rolipram at low doses enhanced long-term memory in mice29 and also reversed memory deficits observed in APP/PS1 transgenic mouse.19 PDE appears as the main target of YTX in previous studies, so we analyzed if YTX could modify PDE4 expression in primary cortical neurons derived from 3xTg-AD mice and their wild type littermate. With this purpose, we performed third to seventh div treatments with 1 nM YTX, a concentration that does not affect cellular viability even in chronic exposures (107.2 ± 2.8% mitochondrial function versus nontreated cells). So, YTX was added to the extracellular medium from third to seventh div and cellular lysates were processed for immunochemical analysis. First, we studied PDE4 expression in 3xTg-AD and NonTg neurons and observed (Figure 2) that there were no differences in PDE4 expression between transgenic and nontransgenic neurons, but while YTX did not have any effect over transgenic neurons, it increased PDE4 levels in a 63.6 ± 19.8% in NonTg neurons. In view of these effects, cAMP levels after exposure of cortical neurons to the toxin were also evaluated as previously described in lymphocytes.14 In this case, two different conditions were analyzed, a chronic exposure to 1 nM YTX from third to seventh div and an acute exposure of 30 min to 0.5, 1, and 2 nM YTX. cAMP measurements were made using a competitive enzyme immunoassay (Amersham cAMP BiotrakEIA System, GE Healthcare), but none of the conditions resulted in a clear effect of YTX at this concentration in cAMP basal levels (data not shown).
Figure 2.
Chronic YTX treatment did not modify the steady-state levels of PDE4 in 3xTg-AD neurons but increased it in NonTg neurons. (A) Quantitative analysis of the effect of YTX on PDE4 levels as obtained from three independent experiments showing a significative increase of PDE4 levels in NonTg treated neurons. (B) Representative experiment showing Western blot bands for PDE4 levels in NonTg and 3xTg-AD neurons and in neurons incubated with 1 nM YTX. Results are mean ± SEM of three experiments, each performed in duplicate. Percentages were calculated using the basal PDE4 expression of NonTg neurons as 100% control. *p < 0.05 versus NonTg neurons without treatment.
Yessotoxin Effects over AD Related Pathology
We tested if subtoxic concentrations of YTX could be modifying the Aβ or tau pathology observed in a 3xTg-AD in vitro model that overexpress both hallmarks.30 So, 1 nM YTX from third to seventh div was used. After this, cells were lysed and processed for immunoblotting analysis. As can be seen in Figure 3A, confocal images of 3xTg-AD cells incubated with YTX showed a decrease in the immunoreactivity for 6E10 antibody, which reacts with the abnormally processed isoforms and precursors forms of the Aβ peptide. This decrease was 52.8 ± 2.3% (p = 0.01) versus nontreated 3xTg-AD neurons. Extracellular Aβ levels were also measured by using an ELISA kit. In this cellular model, it was proved that 3xTg-AD presented an increase in amyloid release to the extracellular medium,30 but YTX pretreatment did not have any effect over this measure (data not shown). The other cellular pathology observed in this AD model is the overexpression of phosphorylated tau isoforms. With the aim of studying the effect of YTX on tau pathology, NonTg, 3xTg-AD, and 3xTg-AD-treated lysed neurons were incubated with AT8 (phospho-tau S199/S202/T205) and AT100 (phospho-tau S212/T214) antibodies. Figure 3B shows representative Western blot bands of cortical neurons revealed with the AT8 antibody and the quantitative analysis of the bands which indicates that AT8 expression was decreased by a 28.8 ± 8.02% in YTX-treated neurons versus nontreated 3xTg-AD neurons (p = 0.006). Similarly, AT100 immunoreactivity (Figure 3C) decreased by 22.4 ± 10.8% (p = 0.05) in YTX-treated neurons versus nontreated 3xTg-AD. In all these experiments, NonTg neurons were processed together with 3xTg-AD to ensure that 3xTg-AD overexpression is correct.
Figure 3.
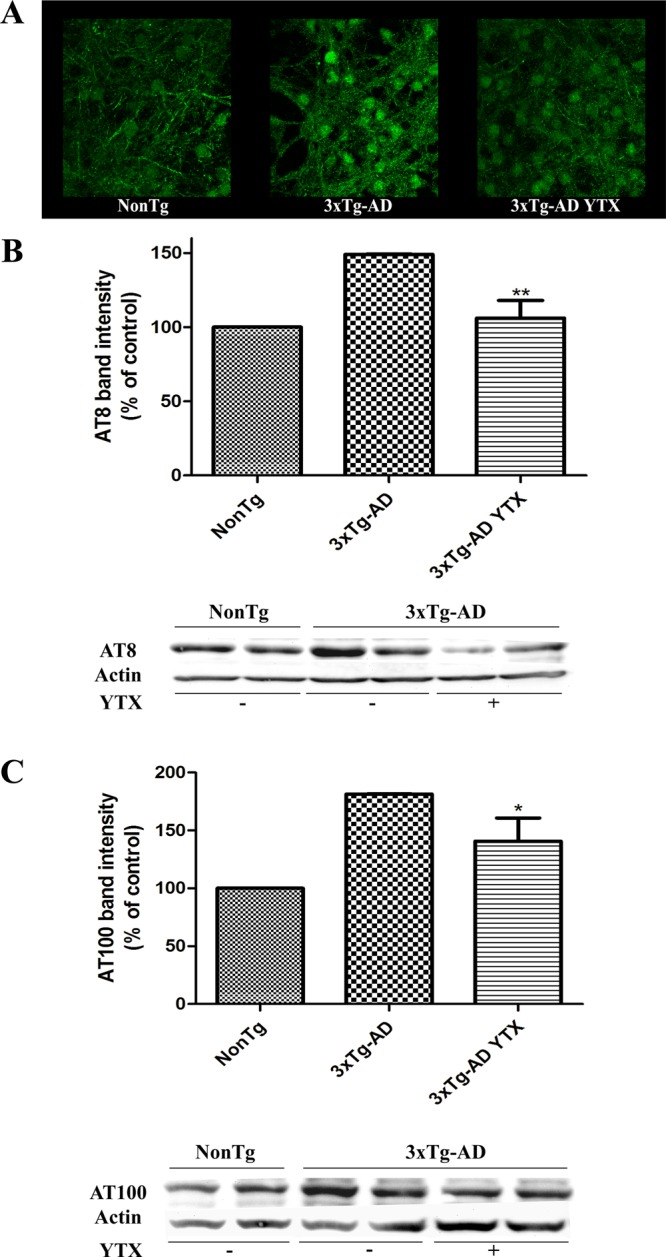
Chronic YTX exposure decreases intracellular Aβ accumulation and tau phosphorylation. (A) Representative confocal images of NonTg, 3xTg-AD, and 3xTg-AD treated neurons incubated with the Aβ40–42 antibody, showing a diminished stain in 3xTg-AD treated neurons. (B) Representative Western blot bands with the AT8 antibody (tau phosphorylated at Ser 199 and Ser 202) in control NonTg, 3xTg-AD, and YTX-treated 3xTg-AD neurons. Quantitative analysis of AT8 levels showing a significative decrease in AT8 levels after YTX treatment. (C) Representative Western blot bands indicating phospho-tau levels in control NonTg, 3xTg-AD, and YTX-treated 3xTg-AD neurons with the AT100 antibody (tau phosphorylated at Ser 212 and Thr 214). Quantification of AT100 levels showing a marked decrease in AT100 band intensity in 3xTg-AD neurons after YTX treatment. Results are mean ± SEM of three experiments, each performed in duplicate. Percentages were calculated using the basal AT8 or AT100 expression of NonTg neurons as 100% control. **p < 0.01 versus 3xTg neurons. *p < 0.05 versus 3xTg-AD neurons.
Study of Kinase Pathways Related with Yessotoxin Effects
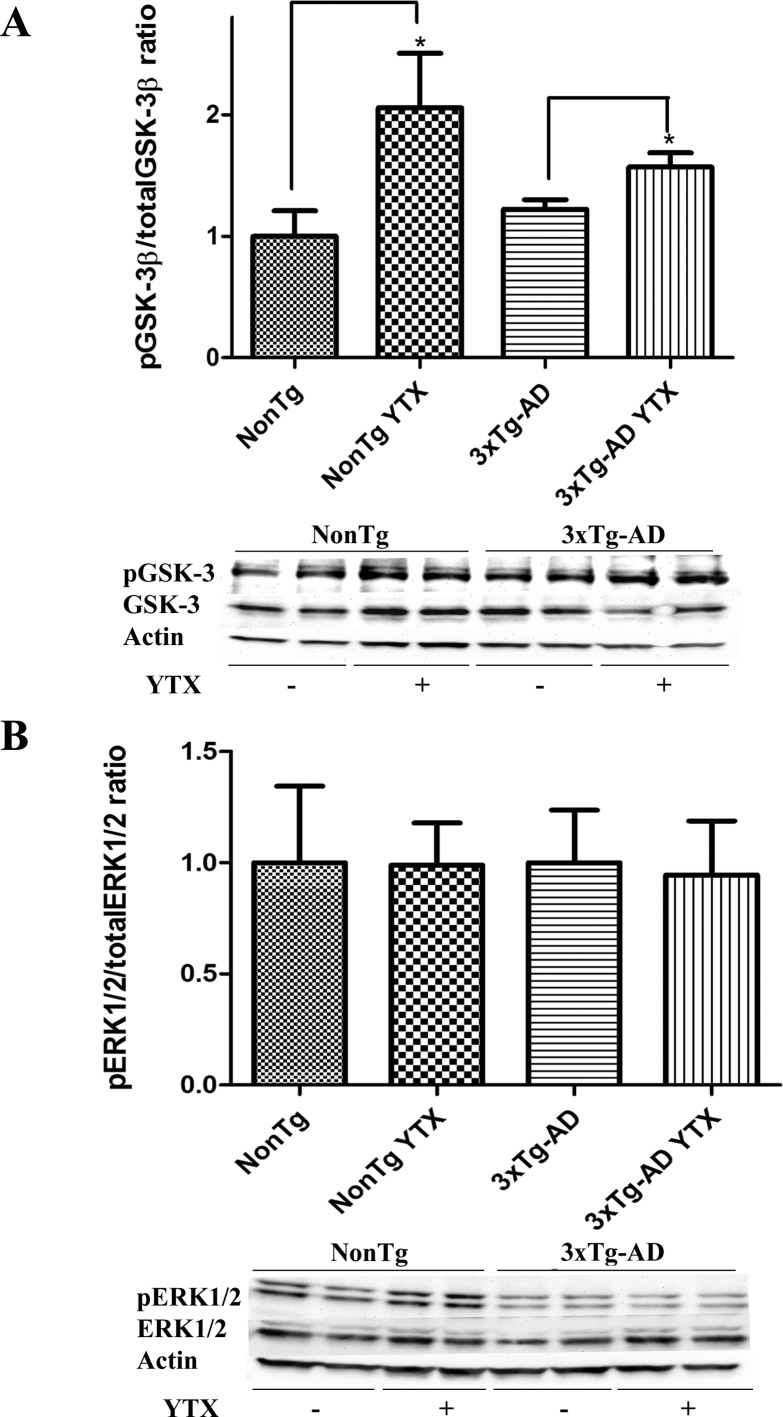
In view of the observed actions of YTX over Aβ and tau pathology, we studied some of the known mechanisms that regulate or affect these two AD hallmarks. In this sense, it is now widely accepted that the abnormal activation of several kinase pathways, including glycogen-synthase kinase-3β (GSK-3β) and extracellular regulated kinase (ERK1/2), are involved in the neurodegenerative progression of AD.31−33 In order to evaluate the role of GSK-3β on the beneficial effects of YTX against AD pathology, GSK-3β expression was analyzed. Western blot with both, phospho-GSK-3β antibody, which recognizes the enzyme phosphorylated in Ser9 and represents the inactive isoform, and total GSK-3β were made. As shown in Figure 4A, yessotoxin increased the expression of the phosphorylated isoform of GSK-3β while total GSK-3β was unaffected, increasing the phospho/total GSK-3β ratio by 51.4 ± 15.1% in NonTg neurons treated with YTX and by a 35.6 ± 4.5% in 3xTg-AD treated neurons (Figure 4A). Additionally, since Aβ can also increase ERK phosphorylation, we studied the possible interactions of this kinase in the YTX mechanism. However, as can be seen in Figure 4B, there was no effect of YTX treatment in phospho/total ERK1/2 ratio. The expression of the phosphorylated isoform was not affected by YTX treatment, and the levels of total ERK1/2 protein were only affected in 3xTg-AD neurons, but this change did not modify the final ratio. We also observed lower levels of both phospho and total kinases in 3xTg-AD levels versus NonTg neurons.
Figure 4.
Effect of YTX exposure on GSK-3 and ERK levels. (A) Chronic YTX treatment increases phosphoGSK-3 expression in 3xTg-AD neurons. Representative experiment showing Western blot bands indicating phospho-GSK-3 (Ser9) and GSK-3 total expression in NonTg and 3xTg-AD neurons alone or treated with YTX and the corresponding quantification of Western blot band intensities showing an increase in phospho-GSK-3/total GSK-3 ratio in NonTg and 3xTg-AD treated neurons versus nontreated primary cortical neurons as obtained from three independent experiments. *p < 0.05. (B) ERK 1/2 expression was not modified after YTX treatment. Representative Western blot bands probed with phospho-ERK1/2 and total ERK antibodies in NonTg, 3xTg-AD, and YTX treated NonTg and 3xTg-AD neurons with the corresponding histogram of the quantification obtained from four independent experiments, each performed in duplicate.
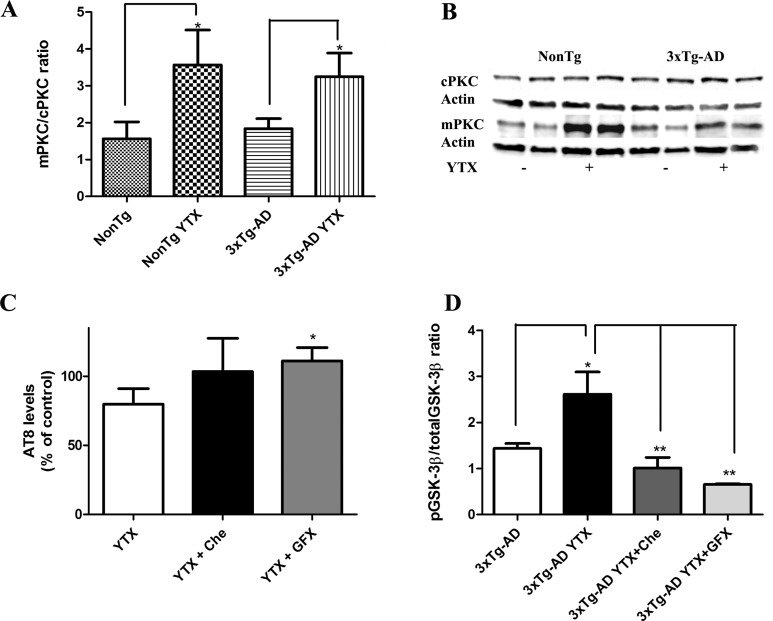
Several reports indicated that GSK-3β can be inactivated by PKC pathways, showing that PKC activation inhibits GSK-3β34,35 and that this process is followed by tau aggregation.36 Therefore, we studied if PKC could mediate the YTX effects in tau and GSK-3β. Cytosol and membrane lysates samples from NonTg and 3xTg-AD cortical neurons treated with YTX and from nontreated neurons were processed for PKC translocation analysis. A relative increase in the ratio membranous/cytosolic fractions was observed in 3xTg-AD treated neurons compared with nontreated cells with an antibody which recognizes classics PKC isoforms (α, β, and γ) (Figure 5). Results show that membranous/cytosolic PKC ratio increased by a 56.18 ± 10.03 in NonTg treated neurons versus nontreated, and increased by 43.52 ± 6.9% in 3xTg-AD treated with YTX versus nontreated 3xTg-AD.
Figure 5.
YTX treatment activates PKC in primary cortical neurons. (A) Corresponding quantification of Western blot band intensities showing an increase in membrane/cytosol ratio in treated neurons versus nontreated primary cortical neurons as obtained from four independent experiments indicating a PKC translocation. (B) Representative experiment showing Western blot bands indicating PKC levels in cytosol (cPKC) and membrane (mPKC) fraction samples in NonTg and 3xTg-AD neurons alone or treated with YTX. Results are expressed as percentage of control cells (nontransgenic neurons). (C) Quantification of AT8 levels in 3xTg-AD neurons treated with YTX alone, YTX plus Chelerytrine (Che) or YTX plus GF109203X (GFX), showing an increase of AT8 expression with YTX+GFX coincubation. Percentages were calculated using the AT8 expression of 3xTg-AD neurons as 100% control. (D) Quantification of GSK-3β levels in 3xTg-AD neurons treated with YTX alone, YTX plus Che, or YTX plus GFX, showing a complete blockage of the increased phospho/total GSK-3β ratio induced by YTX in the presence of Che and GFX. *p < 0.05. **p < 0.01. Results are mean ± SEM of six experiments, each performed in duplicate.
We studied if the blockage of PKA or PDE4 inhibition could be participating in YTX induced phosphorylated tau decrease. So H89, a potent and selective inhibitor of cAMP dependent PKA, 5 μM and 10 μM Rolipram were coincubated with YTX. Immunoblotting assays with AT8 antibody showed that neither H89 nor Rolipram inhibited YTX-induced AT8 decrease (data not shown). As YTX produced PKC activation, we analyzed if 50 nM GF 109203X, a highly selective PKC inhibitor, and 250 nM Chelerythrine, another PKC inhibitor, could modulate YTX effects over AT8 expression. As it is shown in Figure 5C, coincubation with Chelerythrine inhibited YTX effects but the effect was more potent with the PKC inhibitor GF 109203X (p = 0.03).
To strengthen the relationship between YTX effects and PKC modulation, we analyzed if these two PKC blockers, Chelerytrine and GF 109203X, would be able to inhibit also YTX effects over GSK-3 β. As can be observed in Figure 5D, coincubation with the two PKC modulators inhibited the increase in the phospho/total GSK-3β ratio induced by 1 nM YTX (p = 0.004). Once again, the effect was more emphasized in the case of GF 109203X. Furthermore, MTT assays were performed with both PKC blockers and three concentrations of YTX, 1, 5, and 10 nM, as we previously did with other kinase modulators. Both compounds elicited a full recovery of mitochondrial function when they were incubated with 5 nM YTX, reaching values of 108.1 ± 1.14% and 102.5 ± 4.8% versus control nontreated cells for Chelerytrine and GF 109203X, respectively. In the case of the highest concentration tested, 10 nM, neither of the modulators achieved a full cellular viability recovery. However, MTT values increased 2.9-fold for Chelerytrine and 2.6-fold for GF 109203X versus 10 nM YTX treated cells, achieving values of around 50% cellular viability in the case of the PKC modulators and around 20% in the case of 10 nM YTX alone.
Yessotoxin Effects over VDAC Expression
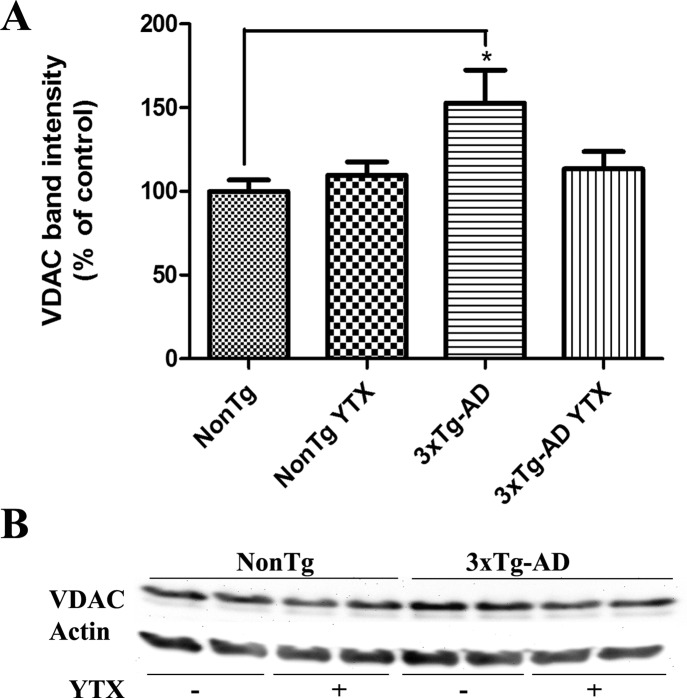
YTX is known to act also through some mitochondrial pathways, showing a potent induced opening of the permeability transition pore (PTP) in the nanomolar range.37,38 Mitochondrial dysfunction acts as a contributor in the pathology of AD with several changes in the activities of different mitochondrial enzymes in early AD progression.39 Several works showed that amyloid precursor protein and amyloid-beta can interact with the mitochondria, resulting in dysfunction.40,41 The voltage-dependent anion channel, VDAC, is an integral mitochondrial protein present also at the neuronal plasma membrane and that participates in Aβ-induced toxicity. In fact, when VDAC antibodies are added before Aβ peptide cell treatment, neuroprotection is observed.42 There is evidence that, as happens in human AD brains, there is an accumulation of VDAC in dystrophic neurites around senile plaques in AD animal models.43 In the same animal model used in the present work, 3xTg-AD mice, 23 different proteins of the mitochondrial proteome were found to have an altered expression between wild type and transgenic mice.44 Among those proteins VDAC-1 and -2 were upregulated in 3xTg-AD cortices. So, we studied the expression of this protein in the primary cortical neurons derived from 3xTg-AD and NonTg fetuses. As it is shown in Figure 6, VDAC expression is upregulated in 3xTg-AD cortical neurons in a 52.7 ± 17.2% (p = 0.017) versus NonTg neurons as previously shown in the animal model. While YTX pretreatment did not show any effect over VDAC expression in NonTg neurons, YTX treatment of 3xTg-AD neurons decreased VDAC band intensity by 23.9 ± 4.9% (p = 0.05) as can be seen in the corresponding histogram (Figure 6A) and the representative Western blot in Figure 6B.
Figure 6.
Chronic YTX effects over VDAC expression. (A) Quantitative analysis of the effect of YTX on VDAC levels as obtained from three independent experiments showing a significative increase in VDAC expression in 3xTg-AD neurons versus NonTg neurons and a decreased expression of VDAC in 3xTg-AD neurons treated with YTX versus nontreated 3xTg-AD. (B) Representative experiment showing Western blot bands for VDAC levels in nontreated NonTg and 3xTg-AD neurons and in the same neurons incubated with 1nM YTX. Results are mean ± SEM of five experiments, each performed in duplicate. Percentages were calculated using the basal VDAC expression of NonTg neurons as 100% control. *p < 0.05.
Several studies have been released about the effects of YTX on the viability of different cell lines and primary cultures,23 with various death pathways and different IC50’s. Perez-Gomez et al. described an IC50 of 20 nM in cerebellar granule neurons,28 but lower doses induced also toxicity signals. We show in the present work that primary cortical neurons are more sensitive to YTX toxicity than the neurons used in the previous study, setting the IC50 for these cells in 4.27 nM. YTX is an apoptotic inducer in mammalian cells,23 but recently another YTX induced death has been reported, named paraptosis.24 This programmed death is caspase-independent, leads to necrosis and appears during development and in some neurodegenerative pathologies.25,26,45 As we have shown, the YTX-induced cell death in primary cortical neurons was caspase-independent since the cell permeable caspase inhibitor Z-VAD did not have any effect over YTX toxicity. The kind of death triggered by YTX, with cellular granulation and swelling, leads us to test if the toxin could be promoting some ionic alterations. Cl– channels are related to the apoptosis mechanism. Cells undergoing apoptosis showed intracellular acidification, and Cl– inhibitors produced neuroprotection against it in neurons.46 The fact that DIDS did not produce any protection over YTX toxicity and even potentiated it could point to ionic alterations induced by this marine phycotoxin. However, DIDS induced a transitory acidification in cells that is recovered within 10 min,47 but in this case it seems that DIDS acidification can be increasing YTX toxicity. In contrast, amiloride was able to partially block YTX induced effects. In the search of a possible pathway mediating the YTX-induced cell death, we studied the involvement of different neurotransmitters. GABAergic modulators did not show any effect, but glutamatergic antagonists were able to slightly inhibit the toxicity elicited by 5 nM YTX, although they failed with higher doses, indicating a partial or indirect relationship of the glutamatergic system in the YTX toxicity or that YTX induced an excitotoxicity process in primary cortical neurons. We also studied one of the best known cellular targets of YTX, PDEs, but rolipram, a PDE inhibitor, only partially inhibited the cell death induced by 10 nM YTX. None of the compounds so far evaluated elicited a whole cellular protection.
As previously mentioned, works done in our laboratory14 demonstrated that YTX is an activator of PDEs in the presence of Ca2+, hence decreasing cAMP levels in human lymphocytes. We did not observe any effect of YTX in cAMP levels, but due to the high toxicity of YTX in this cellular model the concentrations of toxin tested here were 1000 times lower than the concentrations previously used. However, there are reports in different cell lines indicating a PDE inhibition by YTX,15 which makes it an interesting compound for AD screening. Taking advantage of the in vitro AD model developed from 3xTg-AD mice,30 the possible effects of YTX over the main pathological hallmarks of this neurodegenerative disease were tested. PDE4 has been shown to be involved in memory processes through activation of PKA and CREB protein.21 Low doses of rolipram, which did not produce significant effects in the basal levels of cAMP, as happens with YTX in the present work, enhanced long-term memory in mice,29 reversing also memory deficits in APP/PS1 transgenic mouse.19 In this in vitro model of AD, incubation of the neurons from third to seventh div in the presence of 1 nM YTX reduced intracellular Aβ accumulation and tau hyperphosphorylation recognized by AT8 and AT100, two widely used antibodies in tau pathology screening. This effect over phosphorylated tau was not affected by PKA or PDE4 inhibition, pointing to that in this case these two kinases are not involved in YTX effects, the opposite of previous works.14 The decrease in both AD hallmarks was accompanied by an increase in the inactive isoform of GSK-3β, a kinase that phosphorylates several substrates involved in cellular signaling and that has been implicated in the abnormal phosphorylation of tau in AD31,48 which is also activated by Aβ.49 GSK-3 can be inhibited by PKC activation,34 leading to a reduction in AD pathology. The data presented in this work are in agreement with recent works which suggest that YTX action can be mediated through PKC activation, since we described a translocation of PKC classic isoforms from cytosol to membrane fractions, which confirms a PKC activation mediated by YTX in primary cortical neurons. PKC is widely expressed in neurons and interacts with several neuronal system such as cholinergic or GABAergic systems. It participates in learning and memory and an impairment, when PKC cascades are interrupted, it is produced.50 PKC dysfunctions are found in AD, showing PKC levels reduction by Aβ through its binding to PKC, with the consequent inhibition and degradation of the kinase.11 Several reports about the positive effects of PKC activators in AD have been released, based on the fact that they increase soluble amyloid precursor protein and decrease Aβ40–42 accumulation in transgenic mouse brains.12,13 We reported here that YTX effects over tau pathology and over GSK-3β are blocked by PKC inhibitors, confirming the relationship between this marine phycotoxin and PKC.
The results presented here show that YTX can be an interesting molecule for the treatment of AD due to its effects as a PKC activator, resulting in a decrease in tau and Aβ pathology through an interaction with GSK-3 mediated by PKC activation. However, the specific mechanism through which YTX activates PKC and the specific isoforms involved are still unknown. Hence, further studies about the YTX effects in PKC activation and the possible implications in AD therapies will be needed.
Methods
Primary Cortical Neurons
Two colonies of homozygous triple transgenic (3xTg-AD) mice and wild type nontransgenic (NonTg) mice were established at the animal facilities of the University of Santiago de Compostela, Spain, to obtain primary cultures of cortical neurons. All protocols described in this work were revised and authorized by the University of Santiago de Compostela Institutional animal care and use committee.
Primary cortical neurons were obtained from embryonic day 15–17 mice fetuses as recently described elsewhere.30,51,52 Briefly, cerebral cortex was removed and neuronal cells were dissociated by trypsinization followed by mechanical trituration in DNase-containing solution (0.005% w/v) with a soybean trypsin inhibitor (0.05% w/v) at 37 °C. After that, cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with p-amino benzoic acid, insulin, penicillin, and 10% fetal calf serum. The cell suspension was seeded in multiwell plates precoated with poly-d-lysine and incubated for 7–10 days in vitro (div) in a humidified 5% CO2/95% air atmosphere at 37 °C. Cytosine arabinoside, 20 μM, was added before 48 h in culture to prevent growth of non-neuronal cells. In all the experiments, cortical neurons from NonTg and 3xTg-AD mice were prepared and processed simultaneously.
Chemicals and Solutions
Plastic tissue-culture dishes were obtained from Falcon (Madrid, Spain). Fetal calf serum was purchased from Gibco (Glasgow, U.K.), and DMEM was from Biochrom (Berlin, Germany). All other chemicals were reagent grade and purchased from Sigma-Aldrich (Madrid, Spain).
Pure YTX (≥97.9% purity) was obtained from Cifga (Lugo, Spain). Dimethyl sulfoxide (DMSO) was used for the preparation of stock solutions. The final DMSO concentration in the extracellular culture medium was always lower than 0.05%.
Determination of Cellular Viability
Cell viability was assessed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) test, as previously described.53 This test measures mitochondrial function to assess cell viability, showing a good correlation between a drug-induced decrease in mitochondrial activity and its cytotoxicity in neuronal cells.54 The assay was performed in cultures grown in 96-well plates exposed to different compounds added to the culture medium. Cultures were maintained at 37 °C in humidified 5% CO2/95% air atmosphere. Saponine was used as cellular death control. After the exposure time, cells were rinsed and incubated for 60 min with a solution of MTT (500 μg/mL) dissolved in Locke’s buffer containing (in mM): 154 NaCl, 5.6 KCl, 1.3 CaCl2, 1 MgCl2, 5.6 glucose, and 10 HEPES, pH 7.4 adjusted with Tris. After washing off excess MTT, cells were disaggregated with 5% sodium dodecyl sulfate and absorbance of the colored formazan salt was measured at 590 nM in a Syngene plate reader.
Western Blotting
Cultured neurons pretreated with YTX from the third (3rd) to the seventh (7th) div were lysed in 50 mM Tris-HCl-1% Triton-x100 buffer (pH 7.4) containing a complete phosphatase/protease inhibitors cocktail (Roche). The protein concentration was determined by Bradford assay. Samples of cell lysates containing 20 μg of total protein were resolved in gel loading buffer (50 mM Tris-HCl, 100 mM dithiotreitol, 2% SDS, 20% glycerol, 0.05% bromophenol blue, pH 6.8) by SDS-PAGE and transferred onto PVDF membranes (Millipore). The Snap i.d protein detection system was used for blocking and antibody incubation as previously described.30 For the analysis of the membranous and cytosolic fractions, the following protocol was used: membrane samples were obtained from the remaining cellular pellets after the removal of the soluble fraction (cytosolic). Then, the same buffer with Triton X-100 (1.0%) was used to homogenize samples. Samples were incubated on ice for 30 min, exposed to three cycles of sonication, and centrifugated at 120 000 rpm for 20 min. The supernatant obtained was used as the membranous fraction. Primary antibodies used in this work are summarized in Table 1.
Table 1. List of Antibodies and Dilutions Used.
| antibody | immunogen | host | dilution | source |
|---|---|---|---|---|
| 6E10 | Aa 1–16 of Aβ | mouse | 1:1000 | Signet |
| AT8 | peptide with phospho-S199/S202/T205 | mouse | 1:1000 | Pierce |
| AT100 | peptide with phospho-S212/T214 | mouse | 1:1000 | Pierce |
| phospho-GSK-3β | phosphoepitopes Ser9 of GSK-3β | rabbit | 1:10000 | Millipore |
| GSK-3β | recognizes 47 kD GSK-3β protein | rabbit | 1:1000 | Millipore |
| phospho-ERK1/2 | recognizes 42–44 kD phospho-ERK1/2 | mouse | 1:1000 | Cell Signaling |
| ERK1/2 | recognizes 42–44 kD total ERK1/2 | mouse | 1:1000 | Cell Signaling |
| PDE4A | C-terminal region of PDE4A | rabbit | 1:1000 | Thermo Sc |
| PKC | α, β, γ PKC isoforms; does not cross react with other PKC isoforms | rabbit | 1:1000 | Millipore |
| VDAC | KLH-conjugated linear peptide VDAC | rabbit | 1:1000 | Millipore |
| Actin | C-terminal actin fragment, clone C4 | mouse | 1:20000 | Millipore |
The immunoreactive bands were detected using the Supersignal West Pico chemiluminiscent substrate (Pierce) and the Diversity 4 gel documentation and analysis system (Syngene, Cambridge, U.K.). Chemiluminiscence was measured with the Diversity GeneSnap software (Syngene). β-Actin was used as control for lane loading and to normalize chemiluminiscence values.
ELISA
The amount of amyloid β in the culture medium was measured with the Colorimetric BetaMarkTM x-42ELISA kit (SIGNET) following the protocol indicated by the manufacturer. In all the experiments, culture medium samples were obtained at the same days in vitro from NonTg and 3xTg-AD cultures after the treatment. Optical density was measured at 620 nm in a Syngene plate reader.
Statistical Analysis
All data are expressed as means ± SEM of three or more experiments (each performed in duplicate). Statistical comparison was made by ANOVA with Dunnett’s posthoc analysis or Student’s t test. P values of <0.05 were considered statistically significant.
Acknowledgments
We thank Dr. Frank M. Laferla for his support with 3xTg-AD animals.
From Ministerio de Ciencia y Tecnología, Spain: [SAF2009-12581 (subprograma NEF)], [AGL2009-13581-CO2-01], [ TRA2009-0189, AGL2010-17875]. From Xunta de Galicia, Spain: [GRC 2010/10] and [PGDIT 07MMA006261PR], [PGIDIT (INCITE) 09MMA003261PR], [PGDIT (INCITE) 09261080PR], [2009/XA044], and [10PXIB261254 PR]. From EU VIIth Frame Program: [211326-CP (CONffIDENCE)], [265896 BAMMBO], [265409 μAQUA], and [262649 BEADS], [312184 PharmaSea]. From the Atlantic Area Programme (Interreg IVB Trans-national): [2009-1/117 Pharmatlantic]. Eva Alonso is recipient of a predoctoral fellowship from Fondo de Investigaciones Sanitarias (pFIS), Ministerio de Sanidad y Consumo, Spain.
The authors declare no competing financial interest.
References
- Satake M.; MacKenzie L.; Yasumoto T. (1997) Identification of Protoceratium reticulatum as the biogenetic origin of yessotoxin. Nat. Toxins 5, 164–167. [DOI] [PubMed] [Google Scholar]
- Paz B.; Riobo P.; Fernandez M. L.; Fraga S.; Franco J. M. (2004) Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 44, 251–258. [DOI] [PubMed] [Google Scholar]
- Miles C. O.; Wilkins A. L.; Hawkes A. D.; Selwood A. I.; Jensen D. J.; Munday R.; Cooney J. M.; Beuzenberg V. (2005) Polyhydroxylated amide analogs of yessotoxin from Protoceratium reticulatum. Toxicon 45, 61–71. [DOI] [PubMed] [Google Scholar]
- Ogino H.; Kumagai M.; Yasumoto T. (1997) Toxicologic evaluation of yessotoxin. Nat. Toxins 5, 255–259. [DOI] [PubMed] [Google Scholar]
- Takai A. (1988) Okadaic acid. Protein phosphatase inhibition and muscle contractile effects. J. Muscle Res. Cell Motil. 9, 563–565. [DOI] [PubMed] [Google Scholar]
- Martín López A.; Gallardo Rodriguez J.; Sanchez Mirón A.; F. G. C.; Molina Grima E. (2011) Immunoregulatory potential of marine algal toxins yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicol. Lett. 207, 167–172. [DOI] [PubMed] [Google Scholar]
- Alkon D. L.; Epstein H.; Kuzirian A.; Bennett M. C.; Nelson T. J. (2005) Protein synthesis required for long-term memory is induced by PKC activation on days before associative learning. Proc. Natl. Acad. Sci. U.S.A. 102, 16432–16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H.; Hara C.; Yamaguchi K.; Miyawaki A. (2004) PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron 41, 405–415. [DOI] [PubMed] [Google Scholar]
- Rossi M. A.; Mash D. C.; deToledo-Morrell L. (2005) Spatial memory in aged rats is related to PKCgamma-dependent G-protein coupling of the M1 receptor. Neurobiol. Aging 26, 53–68. [DOI] [PubMed] [Google Scholar]
- Cole G.; Dobkins K. R.; Hansen L. A.; Terry R. D.; Saitoh T. (1988) Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 452, 165–174. [DOI] [PubMed] [Google Scholar]
- Cordey M.; Gundimeda U.; Gopalakrishna R.; Pike C. J. (2003) Estrogen activates protein kinase C in neurons: role in neuroprotection. J. Neurochem. 84, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Sun M. K.; Alkon D. L. (2010) Pharmacology of protein kinase C activators: cognition-enhancing and antidementic therapeutics. Pharmacol. Ther. 127, 66–77. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R.; Tan M.; Dewachter I.; Kuiperi C.; Van der Auwera I.; Wera S.; Qiao L.; Bank B.; Nelson T. J.; Kozikowski A. P.; Van Leuven F.; Alkon D. L. (2004) Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 101, 11141–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso A.; de la Rosa L.; Vieytes M. R.; Yasumoto T.; Botana L. M. (2003) Yessotoxin, a novel phycotoxin, activates phosphodiesterase activity. Effect of yessotoxin on cAMP levels in human lymphocytes. Biochem. Pharmacol. 65, 193–208. [DOI] [PubMed] [Google Scholar]
- Campas M.; de la Iglesia P.; Fernandez-Tejedor M.; Diogene J. (2010) Colorimetric and electrochemical phosphodiesterase inhibition assays for yessotoxin detection: development and comparison with LC-MS/MS. Anal. Bioanal. Chem. 396, 2321–2330. [DOI] [PubMed] [Google Scholar]
- Dell’Ovo V.; Bandi E.; Coslovich T.; Florio C.; Sciancalepore M.; Decorti G.; Sosa S.; Lorenzon P.; Yasumoto T.; Tubaro A. (2008) In vitro effects of yessotoxin on a primary culture of rat cardiomyocytes. Toxicol. Sci. 106, 392–399. [DOI] [PubMed] [Google Scholar]
- Hunter R. W.; Mackintosh C.; Hers I. (2009) Protein kinase C-mediated phosphorylation and activation of PDE3A regulate cAMP levels in human platelets. J. Biol. Chem. 284, 12339–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. E.; Webley G. E. (1991) Prostaglandin F2 alpha stimulates cAMP phosphodiesterase via protein kinase C in cultured human granulosa cells. Mol. Cell. Endocrinol. 82, 207–214. [DOI] [PubMed] [Google Scholar]
- Gong B.; Vitolo O. V.; Trinchese F.; Liu S.; Shelanski M.; Arancio O. (2004) Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 114, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo O. V.; Sant’Angelo A.; Costanzo V.; Battaglia F.; Arancio O.; Shelanski M. (2002) Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. U.S.A. 99, 13217–13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U.; Huang Y. Y.; Kandel E. R. (1993) Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260, 1661–1664. [DOI] [PubMed] [Google Scholar]
- Bailey C. H.; Bartsch D.; Kandel E. R. (1996) Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. U.S.A. 93, 13445–13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsnes M. S.; Espenes A. (2011) Yessotoxin as an apoptotic inducer. Toxicon 57, 947–958. [DOI] [PubMed] [Google Scholar]
- Korsnes M. S.; Espenes A.; Hetland D. L.; Hermansen L. C. (2011) Paraptosis-like cell death induced by yessotoxin. Toxicol. In Vitro 25, 1764–1770. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C.; Gurney M. E. (1994) Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 145, 1271–1279. [PMC free article] [PubMed] [Google Scholar]
- Turmaine M.; Raza A.; Mahal A.; Mangiarini L.; Bates G. P.; Davies S. W. (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. U.S.A. 97, 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini A.; Marchesini E.; Poletti R.; Ottaviani E. (2004) Acute toxic effect of the algal yessotoxin on Purkinje cells from the cerebellum of Swiss CD1 mice. Toxicon 43, 347–352. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez A.; Ferrero-Gutierrez A.; Novelli A.; Franco J. M.; Paz B.; Fernandez-Sanchez M. T. (2006) Potent neurotoxic action of the shellfish biotoxin yessotoxin on cultured cerebellar neurons. Toxicol. Sci. 90, 168–177. [DOI] [PubMed] [Google Scholar]
- Barad M.; Bourtchouladze R.; Winder D. G.; Golan H.; Kandel E. (1998) Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl. Acad. Sci. U.S.A. 95, 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C.; Alonso E.; Rubiolo J. A.; Vieytes M. R.; LaFerla F. M.; Gimenez-Llort L.; Botana L. M. (2010) Profile for amyloid-beta and tau expression in primary cortical cultures from 3xTg-AD mice. Cell. Mol. Neurobiol. 30, 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I.; Gomez-Isla T.; Puig B.; Freixes M.; Ribe E.; Dalfo E.; Avila J. (2005) Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathie. Curr. Alzheimer Res. 2, 3–18. [DOI] [PubMed] [Google Scholar]
- Phiel C. J.; Wilson C. A.; Lee V. M.; Klein P. S. (2003) GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptide. Nature 423, 435–439. [DOI] [PubMed] [Google Scholar]
- Young K. F.; Pasternak S. H.; Rylett R. J. (2009) Oligomeric aggregates of amyloid beta peptide 1–42 activate ERK/MAPK in SH-SY5Y cells via the alpha7 nicotinic receptor. Neurochem. Int. 55, 796–801. [DOI] [PubMed] [Google Scholar]
- Fang X.; Yu S.; Tanyi J. L.; Lu Y.; Woodgett J. R.; Mills G. B. (2002) Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 22, 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie L.; Band C. J.; Kong M.; Bergeron J. J.; Posner B. I. (1999) Regulation of glycogen synthase in rat hepatocytes. Evidence for multiple signaling pathways. J. Biol. Chem. 274, 28279–28285. [DOI] [PubMed] [Google Scholar]
- Cho J. H.; Johnson G. V. (2004) Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J. Biol. Chem. 279, 54716–54723. [DOI] [PubMed] [Google Scholar]
- Bianchi C.; Fato R.; Angelin A.; Trombetti F.; Ventrella V.; Borgatti A. R.; Fattorusso E.; Ciminiello P.; Bernardi P.; Lenaz G.; Parenti Castelli G. (2004) Yessotoxin, a shellfish biotoxin, is a potent inducer of the permeability transition in isolated mitochondria and intact cells. Biochim. Biophys. Acta 1656, 139–147. [DOI] [PubMed] [Google Scholar]
- Korsnes M. S.; Hetland D. L.; Espenes A.; Aune T. (2006) Induction of apoptosis by YTX in myoblast cell lines via mitochondrial signalling transduction pathway. Toxicol. In Vitro 20, 1419–1426. [DOI] [PubMed] [Google Scholar]
- Reddy P. H.; Beal M. F. (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 14, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C.; Wang N.; Yao J.; Sosunov A.; Chen X.; Lustbader J. W.; Xu H. W.; Stern D.; McKhann G.; Yan S. D. (2005) Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s diseas. FASEB J. 19, 2040–2041. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen C. A.; Alikhani N.; Behbahani H.; Wiehager B.; Pavlov P. F.; Alafuzoff I.; Leinonen V.; Ito A.; Winblad B.; Glaser E.; Ankarcrona M. (2008) The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. U.S.A. 105, 13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R.; Ramirez C. M.; Gonzalez M.; Gonzalez-Munoz E.; Zorzano A.; Camps M.; Alonso R.; Diaz M. (2007) Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons. Mol. Membr. Biol. 24, 148–160. [DOI] [PubMed] [Google Scholar]
- Ferrer I. (2009) Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. J. Bioenerg Biomembr. 41, 425–431. [DOI] [PubMed] [Google Scholar]
- Chou J. L.; Shenoy D. V.; Thomas N.; Choudhary P. K.; Laferla F. M.; Goodman S. R.; Breen G. A. (2011) Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. J. Proteomics 74, 466–479. [DOI] [PubMed] [Google Scholar]
- Bredesen D. E.; Rao R. V.; Mehlen P. (2006) Cell death in the nervous system. Nature 443, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.; Xiao A. Y.; Jin C.; Yang A.; Lu Z. Y.; Yu S. P. (2004) Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflugers Arch. 448, 325–334. [DOI] [PubMed] [Google Scholar]
- Himi T.; Ishizaki Y.; Murota S. I. (2002) 4,4′-diisothiocyano-2, 2′-stilbenedisulfonate protects cultured cerebellar granule neurons from death. Life Sci. 70, 1235–1249. [DOI] [PubMed] [Google Scholar]
- Pei J. J.; Braak E.; Braak H.; Grundke-Iqbal I.; Iqbal K.; Winblad B.; Cowburn R. F. (1999) Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 58, 1010–1019. [DOI] [PubMed] [Google Scholar]
- Sul D.; Kim H. S.; Lee D.; Joo S. S.; Hwang K. W.; Park S. Y. (2009) Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 84, 257–262. [DOI] [PubMed] [Google Scholar]
- Takashima A.; Yokota T.; Maeda Y.; Itoh S. (1991) Pretreatment with caerulein protects against memory impairment induced by protein kinase C inhibitors in the rat. Peptides 12, 699–703. [DOI] [PubMed] [Google Scholar]
- Alonso E.; Vale C.; Vieytes M. R.; Laferla F. M.; Gimenez-Llort L.; Botana L. M. (2011) 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Abeta and hyperphosphorylated tau in vitro. Neurochem. Int. 59, 1056–1065. [DOI] [PubMed] [Google Scholar]
- Alonso E.; Vale C.; Vieytes M. R.; Laferla F. M.; Gimenez-Llort L.; Botana L. M. (2011) The cholinergic antagonist gymnodimine improves Abeta and tau neuropathology in an in vitro model of Alzheimer disease. Cell. Physiol. Biochem. 27, 783–794. [DOI] [PubMed] [Google Scholar]
- Vale C.; Nicolaou K. C.; Frederick M. O.; Gomez-Limia B.; Alfonso A.; Vieytes M. R.; Botana L. M. (2007) Effects of azaspiracid-1, a potent cytotoxic agent, on primary neuronal cultures. A structure-activity relationship study. J. Med. Chem. 50, 356–363. [DOI] [PubMed] [Google Scholar]
- Varming T.; Drejer J.; Frandsen A.; Schousboe A. (1996) Characterization of a chemical anoxia model in cerebellar granule neurons using sodium azide: protection by nifedipine and MK-801. J. Neurosci. Res. 44, 40–46. [DOI] [PubMed] [Google Scholar]