Abstract
The purpose of the present study was to evaluate the significance of immunogenetic factors on the survival of pancreatic allografts in beagle dogs. Donors and recipients were leukocyte antigen (DLA)-typed and mixed lymphocyte culture (MLC)-tested. Recipients were made diabetic by total pancreatectomy and immediately implanted intraperitoneally with a vascularized, free-draining (duct unligated) pancreatic segmental (FDPS) allograft. Two groups of dogs were studied. In group I consisting of donor-recipient littermates, recipients were immunosuppressed with prednisone and azathioprine (n = 16 dogs), or not immunosuppressed (n = 4). In group II, recipients were made specifically unresponsive by total body radiation, autologous marrow implantation, and kidney transplantation from DLA-MLC identical donors, 1 yr before FDPS transplantation from the corresponding original kidney donors.
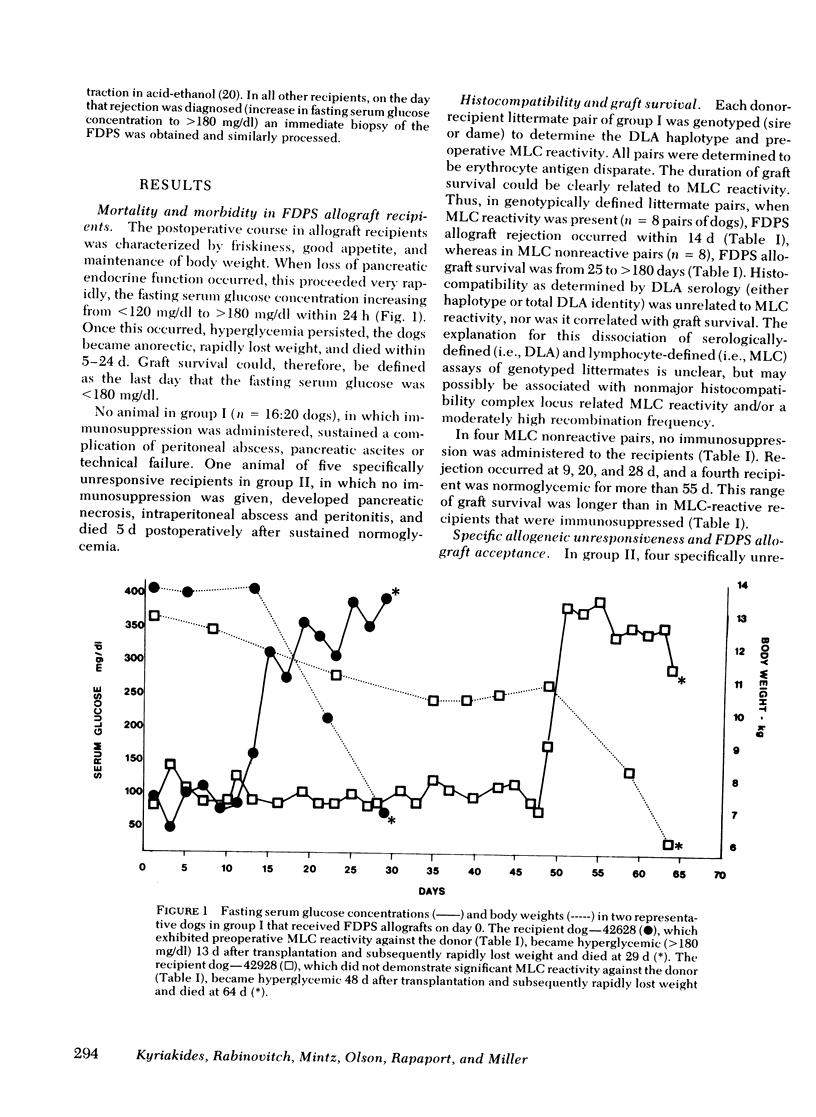
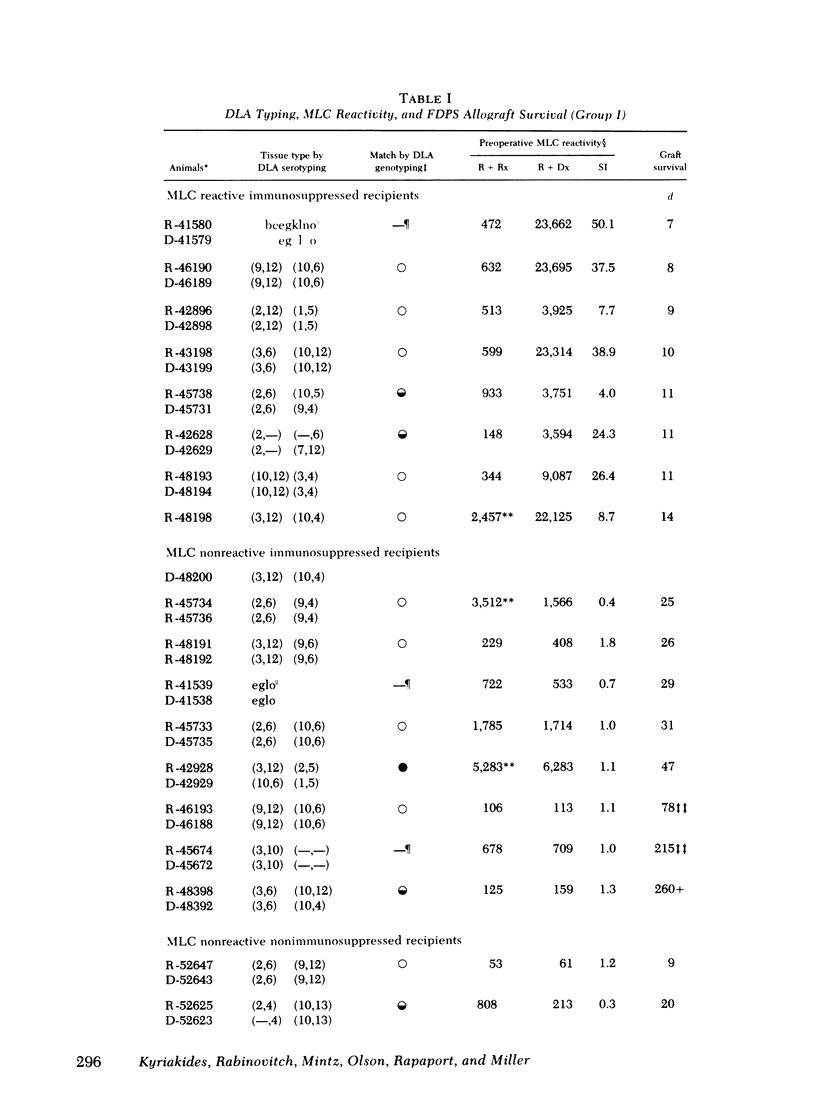
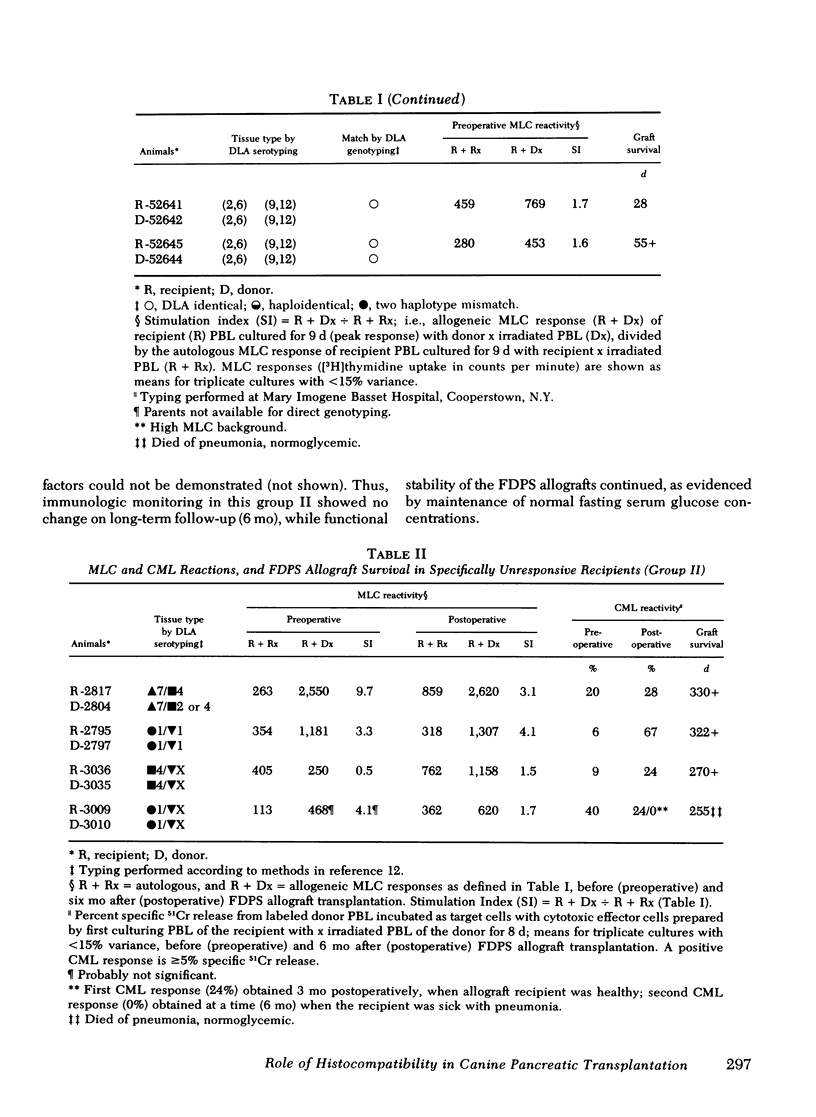
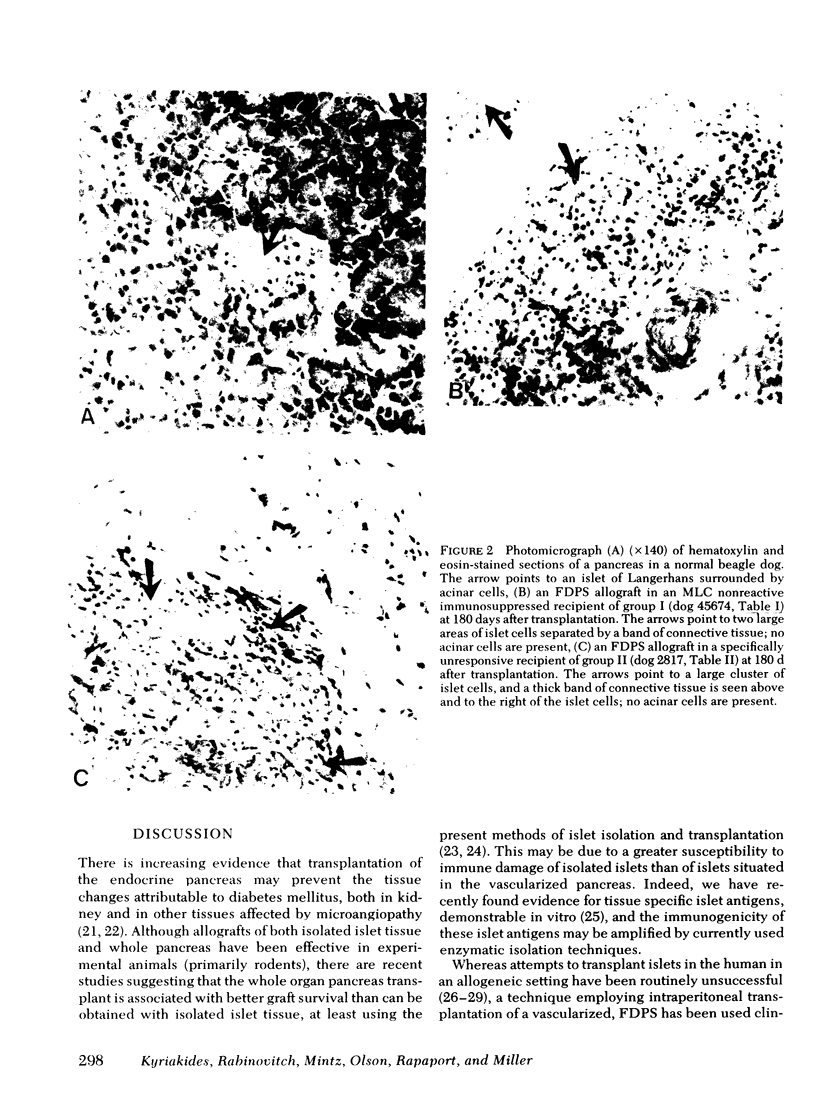
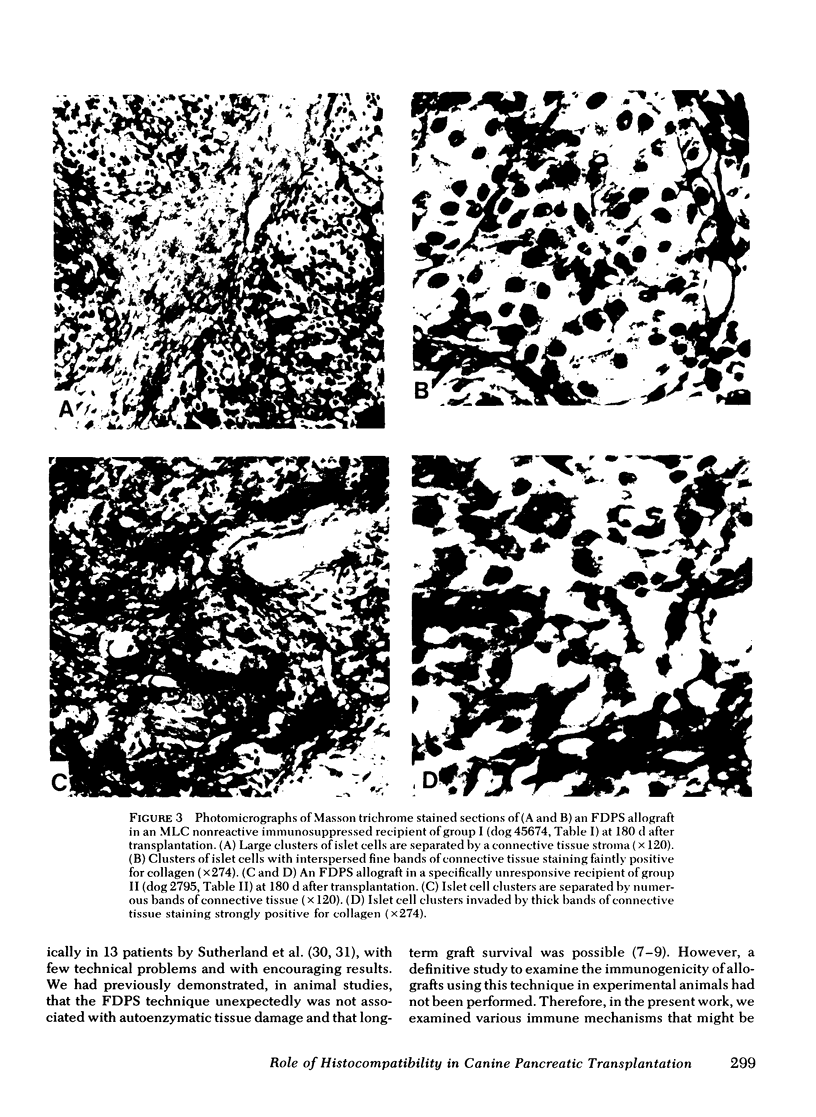
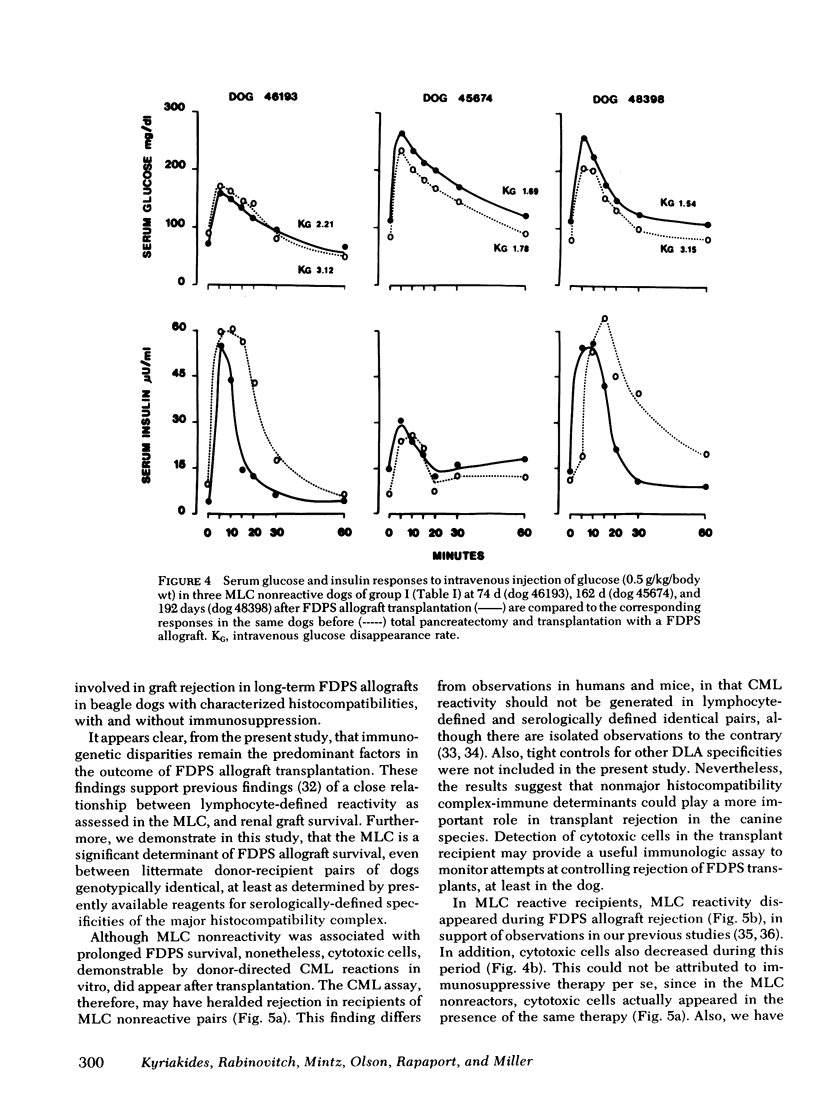
Survival of the FDPS grafts in group I was inversely related to pretransplant MLC reactivity, irrespective of DLA genotyped match between donor and recipient. Thus, immunosuppressed high MLC reactors (n = 8) rejected FDPS grafts between 7 and 14 d, whereas immunosuppressed low MLC reactors (n = 8) accepted grafts for 25 to 260+ days, and nonimmunosuppressed low MLC reactors (n = 4) accepted grafts for 9-55 d. Rejection (hyperglycemia) of FDPS grafts was sudden, permanent, and unpredictable despite weekly intravenous glucose tolerance tests with measurements of glucose disappearance rates and serum insulin responses. Nevertheless, serial in vitro cell-mediated lymphocytotoxicity (CML) assays revealed increases in CML before graft rejection in low MLC reactors, and decreases in both CML and MLC responses before graft rejection in high MLC reactors. FDPS graft survival was indefinite (>6 mo) in group II dogs, despite low-grade MLC reactivity (2:4 dogs) and CML responses (4:4 dogs). Biopsies of FDPS grafts at 6 mo in normoglycemic dogs showed disappearance of exocrine tissue and coalescence of islets in both groups I and II, but with less fibrosis in group I (immunosuppressed).
These results indicate that (a) pancreatic islets in vascularized grafts (FDPS) may survive indefinitely in the presence of a good tissue match best predicted by MLC testing, (b) tissue specific histocompatibility factors appear to be common enough between kidney and pancreas to allow for long-term survival of both organs transplanted from the same donor, at least in appropriate recipients (group II), and (c) immunosuppression is associated with less fibrosis in FDPS allografts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvaroff R., Cannon F. D., Blumenstock D. A., Ferrebee J. W., Rapaport F. T. Mixed leukocyte culture reactivity and the outcome of bone marrow transplantation in dogs. Transplant Proc. 1978 Mar;10(1):119–122. [PubMed] [Google Scholar]
- Barker C. F., Naji A., Silvers W. K. Immunologic problems in islet transplantation. Diabetes. 1980;29 (Suppl 1):86–92. doi: 10.2337/diab.29.1.s86. [DOI] [PubMed] [Google Scholar]
- Dubernard J. M., Traeger J., Neyra P., Touraine J. L., Tranchant D., Blanc-Brunat N. A new method of preparation of segmental pancreatic grafts for transplantation: trials in dogs and in man. Surgery. 1978 Nov;84(5):633–639. [PubMed] [Google Scholar]
- Feighery C., Stastny P. HLA-D region-associated determinants serve as targets for human cell-mediated lysis. J Exp Med. 1979 Feb 1;149(2):485–494. doi: 10.1084/jem.149.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller L., Kyriakides G., Flaa C., Esquenazi V., Miller J. In vitro generation of human mixed lymphocyte culture suppressor cells. I. Cellular characterization and specificity. Transplantation. 1980;29(1):54–60. doi: 10.1097/00007890-198001000-00012. [DOI] [PubMed] [Google Scholar]
- Gliedman M. L., Gold M., Whittaker J., Rifkin H., Soberman R., Freed S., Tellis V., Veith F. J. Clinical segmental pancreatic transplantation with ureter-pancreatic duct anastomosis for exocrine drainage. Surgery. 1973 Aug;74(2):171–180. [PubMed] [Google Scholar]
- Gray B. N., Watkins E., Jr Prevention of vascular complications of diabetes by pancreatic islet transplantation. Arch Surg. 1976 Mar;111(3):254–257. doi: 10.1001/archsurg.1976.01360210048009. [DOI] [PubMed] [Google Scholar]
- Groth C. G., Andersson A., Björkén C., Gunnarsson R., Hellerström C., Lundgren G., Petersson B., Swenne I., Ostman J. Transplantation of fetal pancreatic microfragments via the portal vein to a diabetic patient. Diabetes. 1980;29 (Suppl 1):80–83. doi: 10.2337/diab.29.1.s80. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Hattler B. G., Jr, Miller J. Changes in human mixed lymphocyte culture reactivity as an indicator of kidney rejection. Transplant Proc. 1972 Dec;4(4):655–657. [PubMed] [Google Scholar]
- Hattler B. G., Jr, Miller J., Johnson M. C. Cellular and humoral factors governing canine mixed lymphocyte cultures after renal transplantation. II. Cellular. Transplantation. 1972 Jul;14(1):47–56. doi: 10.1097/00007890-197207000-00007. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Idezuki Y., Feemster J. A., Dietzman R. H., Lillehei R. C. Experimental pancreaticoduodenal preservation and transplantation. Surg Gynecol Obstet. 1968 May;126(5):1002–1014. [PubMed] [Google Scholar]
- Kyriakides G. K., Arora V. K., Lifton J., Nuttall F. Q., Miller J. Porcine pancreatic transplantation. I. Autotransplantation of duct ligated segments. J Surg Res. 1976 May;20(5):451–460. doi: 10.1016/0022-4804(76)90119-0. [DOI] [PubMed] [Google Scholar]
- Kyriakides G. K., Arora V. K., Lifton J., Nuttall F. Q., Miller J. Porcine pancreatic transplants. II. Allotransplantation of duct ligated segments. J Surg Res. 1976 May;20(5):461–466. doi: 10.1016/0022-4804(76)90120-7. [DOI] [PubMed] [Google Scholar]
- Kyriakides G. K., Nuttall F., Miller J. Intraperitoneal segmental pancreatic allografts with unligated ducts in pigs. Transplant Proc. 1979 Mar;11(1):527–529. [PubMed] [Google Scholar]
- Kyriakides G. K., Sutherland D. E., Olson L., Miller J., Najarian J. S. Segmental pancreatic transplantation in dogs. Transplant Proc. 1979 Mar;11(1):530–532. [PubMed] [Google Scholar]
- Kyriakides G., Miller J., Lifton J., Najarian J. S. Effect of steroids on structure and endocrine function of duct-ligated porcine pancreatic autografts. Surg Forum. 1974;25(0):384–386. [PubMed] [Google Scholar]
- Meyer W., Castelfranchi P. L., Ruiz O. J., Aquino C. J., Lillehei R. C. Pancreas allotransplantation without duodenum. J Surg Res. 1972 Feb;12(2):128–137. doi: 10.1016/0022-4804(72)90133-3. [DOI] [PubMed] [Google Scholar]
- Miller J., Hattler B. G., Jr Reactivity of lymphocytes in mixed culture in response to human renal transplantation. Surgery. 1972 Aug;72(2):220–228. [PubMed] [Google Scholar]
- Miller J., Hattler B., Davis M., Johnson M. C. Cellular and humoral factors governing canine mixed lymphocyte cultures after renal transplantation. I. Antibody. Transplantation. 1971 Jul;12(1):65–76. doi: 10.1097/00007890-197107000-00012. [DOI] [PubMed] [Google Scholar]
- Miller J., Lifton J., Rood F., Kyriakides G., Gajl-Peczalska K. Y., Yunis E. J., Hattler B. G., Jr Functional characterization of blocking antibody after human renal transplantation. Surgery. 1974 Jul;76(1):129–141. [PubMed] [Google Scholar]
- Miller J., Lifton J., Wilcox C., DeWolf W. C. The monitoring of canine LD responsiveness using two systems: (1) blocking antibody in second-set renal allografts and (2) modulation of cellular response stimuli. Transplant Proc. 1978 Jun;10(2):395–402. [PubMed] [Google Scholar]
- Perloff L. J., Naji A., Silvers W. K., McKearn T. J., Barker C. F. Vascularized pancreas versus isolated islet allografts: an immunological comparison. Surgery. 1980 Aug;88(2):222–230. [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J. Experimental transplantation and histocompatibility systems in the canine species. Adv Vet Sci Comp Med. 1978;22:195–219. [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J., Mollen N., Hirasawa H., Asano T., Ferrebee J. W. Induction of unresponsiveness to major transplantable organs in adult mammals: a recapitulation of ontogeny by irradiation and bone marrow replacement. Ann Surg. 1979 Oct;190(4):461–473. doi: 10.1097/00000658-197910000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. A., Fisher A. M. THE INSULIN AND THE ZINC CONTENT OF NORMAL AND DIABETIC PANCREAS. J Clin Invest. 1938 Nov;17(6):725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solliday S., Bach F. H. Cytotoxicity: specificity after in vitro sensitization. Science. 1970 Dec 25;170(3965):1406–1409. doi: 10.1126/science.170.3965.1406. [DOI] [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Najarian J. S. Clinical segmental pancreas transplantation without duct anastomosis in diabetic renal allograft recipients. Diabetes. 1980;29 (Suppl 1):10–18. doi: 10.2337/diab.29.1.s10. [DOI] [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Najarian J. S. Intraperitoneal transplantation of immediately vascularized segmental pancreatic grafts without duct ligation. A clinical trial. Transplantation. 1979 Dec;28(6):485–491. [PubMed] [Google Scholar]
- Sutherland D. E., Matas A. J., Goetz F. C., Najarian J. S. Transplantation of dispersed pancreatic islet tissue in humans: autografts and allografts. Diabetes. 1980;29 (Suppl 1):31–44. doi: 10.2337/diab.29.1.s31. [DOI] [PubMed] [Google Scholar]
- Sutherland D. E., Matas A. J., Najarian J. S. Pancreatic islet cell transplantation. Surg Clin North Am. 1978 Apr;58(2):365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- Ting A., Terasaki P. I. Lymphocyte-dependent antibody cross-matching for transplant patients. Lancet. 1975 Feb 8;1(7902):304–306. doi: 10.1016/s0140-6736(75)91209-x. [DOI] [PubMed] [Google Scholar]
- Traeger J., Dubernard J. M., Touraine J. L., Neyra P., Malik M. C., Pelissard C., Ruitton A. Pancreatic transplantation in man: a new method of pancreas preparation and results on diabetes correction. Transplant Proc. 1979 Mar;11(1):331–335. [PubMed] [Google Scholar]
- Uchida H., Ruiz J. O., Castelfranchi P. L., Schultz L. S., Lillehei R. C. New technique of one-stage heterotopic pancreaticoduodenal autotransplantation in dogs. Surgery. 1971 Oct;70(4):604–608. [PubMed] [Google Scholar]
- Usadel K. H., Schwedes U., Bastert G., Steinau U., Klempa I., Fassbinder W., Schöffling K. Transplantation of human fetal pancreas: experience in thymusaplastic mice and rats and in a diabetic patient. Diabetes. 1980;29 (Suppl 1):74–79. doi: 10.2337/diab.29.1.s74. [DOI] [PubMed] [Google Scholar]