Key Points
AIDS-related DLBCL is highly angiogenic with markedly higher blood-vessel density than sporadic cases.
Infiltration by activated cytotoxic cells in AIDS-related DLBCL is determined by the presence of LMP1 and/or p24 viral antigens.
Abstract
Despite the use of highly active antiretroviral therapy (HAART), AIDS-related lymphoma remains common. We investigated the tumor, microenvironment, and viral components in 41 AIDS-related diffuse large B-cell lymphomas (AR-DLBCLs) in the pre- and post-HAART era. The outcome has improved and the frequency of the prognostically unfavorable immunoblastic histology has decreased after HAART. Compared with sporadic cases, AR-DLBCL demonstrated increased hyperproliferation (P < .001) and c-Myc rearrangements, reduced CD4+ (P < .001) and FOXP3+ T cells (P < .001), increased activated cytotoxic cells (P < .001), but no difference in tumor-associated macrophages. Our analysis showed that AR-DLBCL is highly angiogenic with higher blood-vessel density than sporadic cases (P < .001) and highlighted the role of Epstein-Barr virus in angiogenesis. We recognized viral profiles and as a second step examined the reactive cytotoxic cell infiltrates. Our observation of markedly higher numbers of cytotoxic cells in AR-DLBCL with LMP1 and/or p24 compared with cases lacking viral antigens (P < .001) has important clinical implications, implicitly linked to the immunosurveillance theory. Whereas early initiation of HAART should improve immunosurveillance and reduce the incidence of LMP1-positive AR-DLBCL, cases without viral antigens appear able to avoid immunologic reaction and likely require additional strategies to improve surveillance.
Introduction
As we enter the fourth decade of the HIV/AIDS epidemic, the burden of AIDS-related lymphoma (ARL) remains substantial. After the introduction of highly active antiretroviral therapy (HAART), a dramatic decrease in opportunistic infections, Kaposi sarcoma, and primary central nervous system lymphoma (PCNSL) has been seen, but there has been a less marked decline with respect to systemic diffuse large B-cell lymphoma (DLBCL). A meta-analysis reported its incidence at 6.2 cases per 1000 person-years in 1992 and at 3.6 cases per 1000 patient-years in 1999.1 As the survival of people living with HIV increases, malignancy has become a leading cause of death in this population.2,3 Between 25% and 40% of people living with HIV will develop cancer, with ∼10% attributed to ARL; infection-related cancer is likely to become an increasingly important complication of long-term HIV infection.4,5
HIV-1 increases the risk for systemic DLBCL, the most common form of ARL, by 60- to 200-fold.6 The pathogenesis of ARL is linked to the immunosuppression caused by HIV. Notable differences between AIDS-related DLBCL (AR-DLBCL) and DLBCL in the general population include a higher frequency of extranodal disease and prominent association with Epstein-Barr virus (EBV) and human herpesvirus-8 (HHV-8) gammaherpesviruses. The main cellular targets of HIV are the CD4+ T cells, dendritic cells, and cells of monocyte-macrophage lineage.7 HIV does not infect the neoplastic B cells; instead, the increased lymphomagenesis seen in HIV is hinged on its ability to manipulate the host immune system and the microenvironment.
A number of non-neoplastic cells such as reactive T cells, macrophages, and endothelial cells contribute to the tumor microenvironment and play a crucial role in tumor cell biology. Studies in sporadic DLBCL have identified distinct microenvironmental attributes that are predictive of the clinical behavior,8,9 however, there is a lack of studies exploring the characteristics of the non-neoplastic milieu in AR-DLBCL. Taking into consideration the impact of HIV on key microenvironment components and the mechanisms involved in the neoplastic complications of AIDS, we systematically studied the microenvironment in AR-DLBCL and compared it with specimens obtained from immunocompetent DLBCL patients. We hypothesized that there would be differences consistent with the consequences of HIV, the impact of HAART, and the role of oncogenic herpesviruses. An increased understanding of the microenvironment in the 2 periods of the HIV/AIDS epidemic should yield further insights into ARL pathophysiology and indicate new therapeutic strategies.
Methods
Patients
For this retrospective study, we included 41 HIV-1–infected patients diagnosed with AIDS-related systemic DLBCL at St. Bartholomew Hospital between 1991 and 2011 with available formalin-fixed paraffin-embedded tissue suitable for immunohistochemistry from diagnostic biopsies. Cases of Burkitt lymphoma, PCNSL, primary effusion lymphoma, and large cell lymphoma arising in multicentric Castleman disease were excluded. Biopsies were reviewed independently by 2 expert hematopathologists (M.C. and A.M.L.) for confirmation of diagnosis based on current World Health Organization (WHO) criteria.10 Patients had complete staging investigations including examination of bone marrow and cerebrospinal fluid. Twenty-nine patients received a diagnosis of AR-DLBCL in the era of HAART, defined as commencing on January 1, 1997. HAART comprises at least 3 antiretroviral agents in combination. Chemotherapy consisted of an anthracycline-based regimen but only 12 patients (29%) received rituximab-containing chemotherapy. Demographics, AR-DLBCL features, and HIV parameters are given in Table 1.
Table 1.
Patient characteristics
| Patients | Total | Pre-HAART era | HAART era |
|---|---|---|---|
| HIV-1 patients with DLBCL | |||
| Patients, no. (%) | 41 | 12/41 (29.3) | 29/41 (70.7) |
| Median age, y (range) | 40 (22-62) | 37 (29-53) | 40 (22-62) |
| No. of men/no. of women (%) | 36/5 (87.8/12.2) | 12/0 | 24/5 (82.8/17.2) |
| Stage III/IV (%) | 21/41(51.2) | 7/12 (58.3) | 14/29 (48.3) |
| B symptoms (%) | 21/41 (51.2) | 11/12 (91.7) | 10/29 (34.5) |
| ≥1 extranodal site at presentation (%) | 26/41 (63.4) | 8/12 (66.7) | 18/29 (62.1) |
| aaIPI score 2-3 (%)* | 19/40 (47.5) | 9/12 (75) | 10/28 (35.7) |
| Bone marrow infiltration (%) | 7/41 (17.1) | 3/12 (25) | 4/29 (13.8) |
| Cerebrospinal fluid involvement (%) | 5/41 (12.2) | 3/12 (25) | 2/29 (6.9) |
| Known HIV infection prior to AR-DLBCL (%) | 34/41(82.9) | 11/12 (91.7) | 23/29 (79.3) |
| Median time between HIV and AR-DLBCL for known HIV patients, mo (range) | 37.3 (1.37-122.43) | 49.7 (4.53-83.2) | 37 (1.37-122.43) |
| AIDS diagnosis prior to AR-DLBCL, no. (%) | 18/41 (43.9) | 8/12 (66.7) | 10/29 (34.5) |
| Median CD4 count per mm3 (range) | 85.5 (10-840) | 25 (10-150) | 100 (12-840) |
| HIV index 2-4 (%)† | 29/40 (72.5) | 9/11 (81.8) | 20/29 (69) |
| DLBCL patients without HIV | |||
| Patients, no. | 53 | ||
| Median age, y (range) | 53 (19-86) | ||
| No. of men/no. of women (%) | 37/16 (69.8/30.2) | ||
| Stage III/IV (%) | 30/53 (56.6) | ||
| ≥1 extranodal site at presentation (%) | 14/53 (26.4) | ||
| IPI score 4-5 (%)‡ | 9/52 (17.3) | ||
| Bone marrow infiltration (%) | 7/53 (13.2) | ||
aaIPI score includes 3 risk factors: Non-Hodgkin's Lymphoma (NHL) stage III/IV, performance status 2-4, and elevated serum lactic dehydrogenase.
HIV index encompasses 4 risk factors: age > 35 y, NHL stage III/IV, CD4 cell count < 100 per mm3, and injection drug use.
IPI score includes 5 risk factors: age > 60 y, NHL stage III/IV, performance status 2-4, elevated lactic dehydrogenase, and ≥2 extranodal sites.
For comparison, we analyzed 53 patients with DLBCL without HIV or other immunocompromising conditions diagnosed at St. Bartholomew Hospital between 1986 and 2011 with known clinicopathological features, treatment, and outcome (Table 1). The specimens were randomly selected from Barts Cancer Institute Tissue Bank, using the presence of high-quality tissue suitable for immunohistochemistry from the diagnostic biopsy as the only criterion. Biopsies were also reviewed. All patients had an anthracycline-containing regimen and 23 patients (43.4%) received rituximab. Ethical approval was obtained from the institutional review board and written informed consent was given by all patients according to the Declaration of Helsinki.
Histology, TMA construction
For each case, standard hematoxylin-and-eosin (H&E) sections were studied and the tumor morphology recorded as centroblastic, immunoblastic, plasmablastic, or unclassified type as outlined in the WHO 2008 classification. Tissue microarrays (TMAs) were prepared from paraffin blocks using a manual tissue arrayer (Beecher Scientific). Triplicate 1-mm diameter cores were obtained from regions of characteristic morphology rich in malignant cells. The position of the cores taken from the tissue blocks was based on premarked H&E-stained sections. Cores were punched into a recipient paraffin block, cut to 3-μm thick sections, and transferred on glass slides.
Immunohistochemical and immunofluorescent labeling
After dewaxing, blocking in hydrogen peroxide/methanol solution, rehydration, and antigen retrieval, the slides were subjected to immunostaining. Primary antibody reaction was detected using a peroxidase-labeled system (Super Sensitive Polymer-HRP IHC Detection System; BioGenex). To characterize the tumor and evaluate the microenvironment, we selected a panel of monoclonal antibodies in 3 categories: (1) lymphoma panel consisting of CD20, CD10, MUM1, bcl-6, bcl-2, CD138, CD5, Ki67, p53, and PDL1; (2) viral factor panel comprising HIV-1 (p24 and tat), EBV (latent membrane protein 1 [LMP1]), and HHV-8 (LANA1); and (3) microenvironment-associated panel including CD3, CD4, CD8, CD56, CD68, CD163, FOXP3, TIA1, granzyme B, perforin, CD57, CD34, and PD1. Controls for HIV-1 protein expression consisted of persistent generalized lymphadenopathy sections. Using EBV-encoded small RNA (EBER) and LMP1, we classified latent EBV infection as follows: EBER+, LMP1− consistent with latency I and EBER+, LMP1+ consistent with latency II or III. For CD20, CD10, MUM1, bcl-6, bcl-2, CD138, CD5, and PDL1, cases were considered positive if 30% or more of the tumor cells were stained. For p53, we analyzed the percentage of positive tumor cells, taking 50% as a cutoff for overexpression. Hyperproliferation was defined based on Ki67 labeling >80%. Regarding expression of viral proteins, cases were judged to be either positive or negative. Supplemental Table 1 (available on the Blood website) lists the antibodies and immunohistochemical conditions used. To investigate the topographic distribution of macrophages and blood vessels, double staining was carried out using diaminobenzidine (DAB) (brown) for CD34 accompanied by CD68 staining with VIP (purple) substrate from Vector Laboratories. Double staining was also performed for CD34 (DAB) followed by staining for HIV-1 p24 protein (VIP). Counterstaining was developed with Mayer hematoxylin. After antigen retrieval by pressure cooking the slides in citrate, double-immunofluorescent labeling of p24 in conjunction with CD68 was developed with use of fluorochrome-conjugated antibodies.
In situ hybridization for EBV infection
To detect EBERs, in situ hybridization was performed on TMA slides using the EBER1 oligonucleotide probe complementary to the viral nontranslated small RNA. Probing was visualized with the iVIEW blue detection kit (Ventana Medical Systems). Cases were considered positive or negative.
FISH for c-Myc translocation
Given the significance of c-Myc rearrangements in the molecular pathogenesis of AR-DLBCL,6 we performed fluorescence in situ hybridization (FISH) analysis on TMA sections using a break-apart DNA probe (DAKO) specific for c-Myc/8p24 using the Histology FISH Accessory kit (DAKO). The hybridization signal was scored as described.11
Quantitative image analysis and manual counting
The slides were scanned with an Olympus BX61 microscope. A review of cores was performed to examine cellularity and enable valid analyses and comparisons; acellular and fibrotic portions were excluded after digital image processing. Immunostains were quantified using computerized image analysis (Ariol System, Applied Imaging; Genetix) based on pathologist-trained visual parameters and were reviewed independently by hematopathologists. Counting CD3, CD8, FOXP3, CD57, TIA1, perforin, and granzyme-positive cells by image analysis was expressed as a mean cell count per mm2 of tissue. The number of CD4+ as well as FOXP3+ T cells per high-power field (hpf; ×400) was scored manually in 3 hpf per core and the average of the 3 cores was recorded. To enumerate macrophage density, we scored CD68+ cells manually in 3 representative hpf per core. The percentage of macrophages in relation to overall cellularity was reported as an average of the 3 cores. Additionally, we calculated with image analysis the mean percentage the CD68-stained area, an account of the proportion of macrophages in the tumor mass. A similar analysis was done for CD163.
Assessing angiogenesis: counting microvessels and angiogenic sprouts
Blood vessels were highlighted by staining endothelial cells for CD34 antigen. Microvessels were recognized as described.12 Individual microvessels were manually counted on a high-powered field (×400); 5 hpf were assessed within a core and counts were recorded. Microvessel density for each tumor was expressed as a mean microvessel count of the 3 cores. In addition to microvessels, angiogenic sprouts were analyzed independently. Any single, small (8-20 μm), round, endothelial structure lacking lumen or pericyte cuff located within the tumor region was considered a countable sprout.13
Statistical analysis
Categorical variables were summarized by counts and percentages, and continuous variables by medians and ranges or means and SDs. Comparisons between groups were performed using Mann-Whitney and Kruskal-Wallis tests for continuous characteristics and Pearson χ2 and Fisher tests for categorical variables. We used the Spearman rank coefficient for associations between continuous variables. Kaplan-Meier estimators were used for survival outcomes. Group comparisons for survival outcomes were tested with the log-rank method. The Cox proportional hazards model was used for survival analysis. Two-sided P values < .05 were considered significant.
Results
Clinical features
HIV-infected patients were more likely to be male (P = .038), develop lymphoma at younger age (P < .001), and present with extranodal disease (P < .001) than their uninfected counterparts. Compared with sporadic DLBCL, AR-DLBCL showed inferior progression-free (P = .006) and overall survival (P < .001). The outlook was improved after HAART, however, HIV-positive status remained a negative factor for overall survival (P = .012) but not for progression-free survival (P = .1). Univariate analysis revealed clinical factors associated with increased survival after AR-DLBCL including stage I or II disease (P = .004), age-adjusted International Prognostic Index (aaIPI) 0-1 (P < .001), no B-class symptoms (P = .003), no prior AIDS (P = .028), HAART treatment (P = .046), and rituximab-containing chemotherapy (P = .013), whereas CD4 count <100 cells/mm3 at presentation was not statistically significant. Multivariate analysis identified aaIPI 0-1 (P < .001) and rituximab treatment (P = .001) as predictors of survival.
Histologic analysis of tumor cells and c-Myc rearrangements
The aim of the study was to examine the diagnostic biopsies of the AR-DLBCL cases compared with sporadic DLBCL. Among HIV-infected patients, 22% had centroblastic, 48.8% immunoblastic, 9.7% plasmablastic lymphoma, and in the remaining 19.5% the histology was unclassified. Before HAART, immunoblastic DLBCL was seen in all cases whereas after HAART this decreased to 27.6%. Immunoblastic/plasmablastic morphology was associated with an adverse outcome (P < .001). Among patients without HIV, centroblastic DLBCL accounted for 69.7% and immunoblastic for 11.7%, whereas unclassified cytology was noted in 18.6%. Using immunohistochemistry, we studied tumor antigens in both groups (Table 2). The immunophenotypic classification into germinal center (GC) and non-GC types was based on CD10, bcl-6, MUM1, with inclusion of CD138.14,15 Immunophenotype assignment was not statistically associated with survival outcome in these AR-DLBCL cases. Regarding antiapoptotic proteins, bcl-2 positivity and p53 overexpression were found in 44% and 47.2% of AR-DLBCL, respectively. Hyperproliferation was significantly more likely to occur in HIV-infected patients (P < .001). Rearrangements involving c-Myc were identified in 23.5% and 5.6% of HIV-infected and sporadic samples, respectively. c-Myc rearrangement was associated with hyperproliferation (P = .024) but no correlation was detected with lymphoma immunophenotype, morphology, p53, and aaIPI.
Table 2.
Immunohistochemical features and EBV status of tumor cells
| Tumor cell–associated marker | AR-DLBCL total | AR-DLBCL pre-HAART era | AR-DLBCL HAART era | Sporadic DLBCL |
|---|---|---|---|---|
| CD20 positive, % (no./total) | 80.5 (33/41) | 83.3 (10/12) | 79.3 (23/29) | 94.34 (50/53) |
| GC phenotype, % (no./total) | 46.34 (19/41) | 41.67 (5/12) | 48.28 (14/29) | 33.96 (18/53) |
| Non-GC phenotype, % (no./total) | 46.34 (19/41) | 33.33 (4/12) | 51.7 (15/29) | 52.8 (28/53) |
| Unclassifiable phenotype, % (no./total) | 7.3 (3/41) | 25 (3/12) | 0 (0/29) | 13.2 (7/53) |
| Bcl-2 positive, % (no./total) | 43.9 (18/41) | 16.7 (2/12) | 55.2 (16/29) | 58.5 (31/53) |
| Median Ki67, % (range, %) | 85 (40-95) | 78.75 (50-90) | 85 (40-95) | 70 (35-92.5) |
| Ki67 > 80%, % (no./total) | 54.55 (18/33) | 50 (4/8) | 56 (14/25) | 13.2 (7/53) |
| CD5 positive, % (no./total) | 8.82 (3/34) | 0 (0/7) | 11.11 (3/27) | 8.82 (3/34) |
| EBER positive, % (no./total) | 56.1 (23/41) | 91.7 (11/12) | 41.7 (12/29) | 5.7 (3/53) |
Differences in the composition of microenvironment between AR-DLBCL and sporadic DLBCL
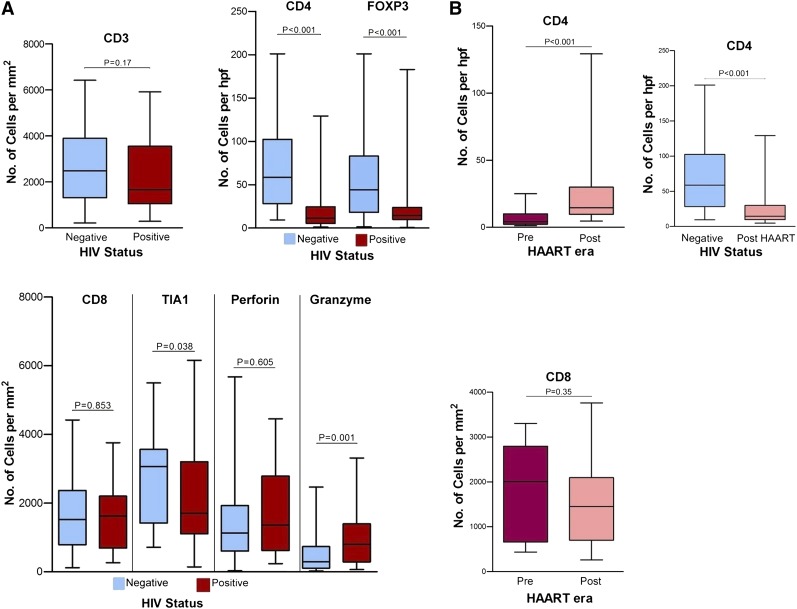
From our current perspective on immunosurveillance, infiltrating lymphocytes play a key role in the host defense against tumor. Specifically, activated cytotoxic and helper T cells are responsible for immune responses within the tumor microenvironment. In the context of AR-DLBCL, we expected distinctive changes in the microenvironment given the abnormalities in cell-mediated immunity caused by HIV. To test this hypothesis, we enumerated immune cells within AR-DLBCL biopsies and compared them to sporadic DLBCL. In fact, AR-DLBCL biopsies contained proportionally fewer but not statistically significant CD3+ T lymphocytes (P = .107) but, as expected, markedly reduced CD4+ (P < .001) and FOXP3+ T cells (P < .001). As a result of CD4 depletion, CD8+ T cells formed the predominant type of the T-cell infiltrate. To identify tumor-infiltrating cytotoxic T lymphocytes (CTLs), we stained for TIA1, perforin, and granzyme B. Comparison of the 2 groups revealed a discrepancy in CTL highlighted by TIA1 and granzyme: TIA1 was more frequent among HIV-negative patients whereas granzyme expression was significantly higher in AR-DLBCL. The physiologic regulation of cytotoxic molecules may help explain this paradox: TIA1 is produced regardless of activation status while granzyme occurs in granules upon cell activation.16,17 Testing for CD56, a characteristic glycoprotein of natural killer (NK) and NKT cells, showed a paucity of positive cells in both groups, ruling out other cytolytic cell types. Thus, AR-DLBCL is characterized by a diminution in the proportion of helper and regulatory T cells and a disproportional enrichment in CTL, especially activated CTL (Figure 1A).
Figure 1.
Comparison of reactive lymphocyte markers. (A) Between HIV-infected (positive) and uninfected (negative) patients. AR-DLBCL contains markedly reduced helper (CD4+) and regulatory (FOXP3+) T cells, whereas evaluation of cytotoxic markers showed that it is characterized by a disproportional enrichment in activated (granzyme-positive) tumor-infiltrating cytotoxic T cells. (B) Between pre-HAART and post-HAART HIV-infected patients. Although loss of infiltrating CD4+ T cells is attenuated after the use of HAART, their number remains significantly lower than in sporadic DLBCL.
Differences in microenvironment between the 2 AIDS periods
The introduction of HAART had a notable impact on AR-DLBCL. Historically, the median survival pre-HAART was <12 months, whereas after HAART it increased to 15 to 34 months.18 This improvement is mainly attributed to the restoration of cell-mediated immunity. As shown in Table 1, HAART-era patients had higher CD4 counts and fewer AIDS-defining conditions than those presenting pre-HAART. We therefore asked whether improvements in cellular immunity were paralleled by changes in the composition of microenvironment. In comparing the data between the 2 periods, we found higher numbers of CD4+ T cells in HAART-era specimens (P < .001) whereas expression of other lymphocytic markers was not significantly different (Figure 1B). We observed only a weak correlation between blood and tissue CD4+ T-cell counts (r = 0.32; P = .049) (data not shown).
Study of tumor-infiltrating macrophages and blood-vessel densities
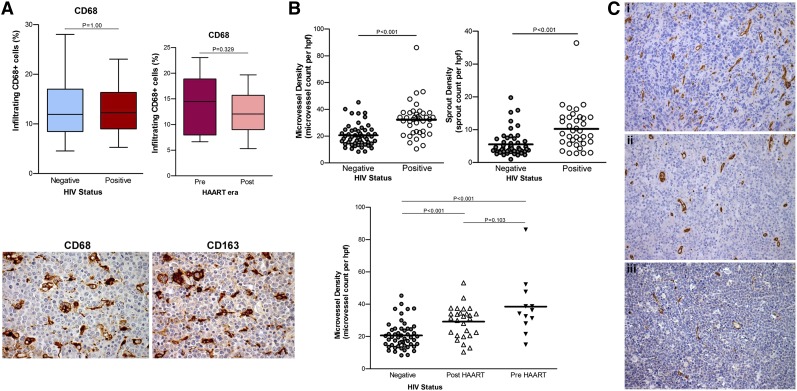
Macrophages are a major target of HIV and a source of virus production.7 Additionally, tumor-associated macrophages play diverse and critical roles in tumorigenesis. We found no significant difference in the number of CD68+ macrophages or in their proportion in tumor mass between HIV-infected and uninfected patients or between the 2 eras (Figure 2A). Although HIV-1 infection strongly represses CD163 expression, orientating macrophages toward a proinflammatory phenotype,19 we noted coordinate expression of CD68 and CD163 in our specimens as illustrated in Figure 2A, suggesting that AR-DLBCL–associated macrophages are alternatively activated (M2). We observed no statistically significant association between the number of macrophages and survival. However, a very poor outcome was noticed in the small number of patients with a very low number of macrophages.
Figure 2.
Tumor-infiltrating macrophages and angiogenesis in AR-DLBCL. (A) The proportion of CD68+ macrophages does not differ significantly between HIV-infected and uninfected patients, and between pre-HAART and post-HAART era patients (top). We noted comparable expression of CD68 and CD163 antigens by tumor-infiltrating macrophages in AR-DLBCL, as shown in this example (bottom). CD163 is a classic marker of anti-inflammatory or alternatively activated (M2) macrophages corresponding to the hemoglobin-haptoglobin scavenger receptor. (B) Microvessel and sprout densities for AR-DLBCL compared with sporadic DLBCL. AR-DLBCL is characterized by a substantially higher amount of neovascularization: the mean (±SD) microvessel and sprout count were 32.2 ± 13.2 per hpf and 10.2 ± 6.3 per hpf, respectively, whereas in sporadic DLBCL the corresponding values were significantly lower, 20.6 ± 8.3 and 5.5 ± 3.7 (P < .001 and P < .001, respectively). (C) Immunohistochemical analysis of CD34+ endothelial cells in 3 representative cases: (i) EBV-positive immunoblastic AR-DLBCL, (ii) EBV-negative centroblastic AR-DLBCL, and (iii) EBV-negative sporadic DLBCL centroblastic type, with respective microvessel counts of 52.22, 29.91, and 16.61 per hpf. All specimens represent lymph node biopsies of hyperproliferative lymphomas without c-Myc translocation. Images were taken with a Leica DM2500 microscope (original magnification, ×200), Leica DFC320 camera.
Despite a body of in vitro evidence indicating a role for endothelial cells in AR-DLBCL biology,20,21 there is a lack of studies examining angiogenesis in clinical specimens. To examine angiogenesis, we assessed the microvessel density and the sprouts, a visible reflection of the dynamic process of angiogenesis.13 Our analysis revealed that the amount of neovasculature was strikingly higher in AR-DLBCL (P < .001 both for microvessels and sprouts) than in sporadic DLBCL (Figure 2B). We next evaluated the relationship between blood-vessel density in AR-DLBCL and CD68+ macrophages but we found no correlation in terms of microvessels (r = 0.175; P = .095) or sprouts (r = 0.174; P = .106). Likewise, no association was seen between microvessels and CD163, lymphocytic infiltration, Ki67, c-Myc status, CD4 count, aaIPI, extranodal involvement, and anatomical site of biopsy. In contrast, microvessels correlated positively with immunoblastic/plasmablastic morphology (P = .007) and EBER positivity (P = .001). Figure 2C illustrates angiogenesis in 3 representative cases.
HIV-1 identification in samples
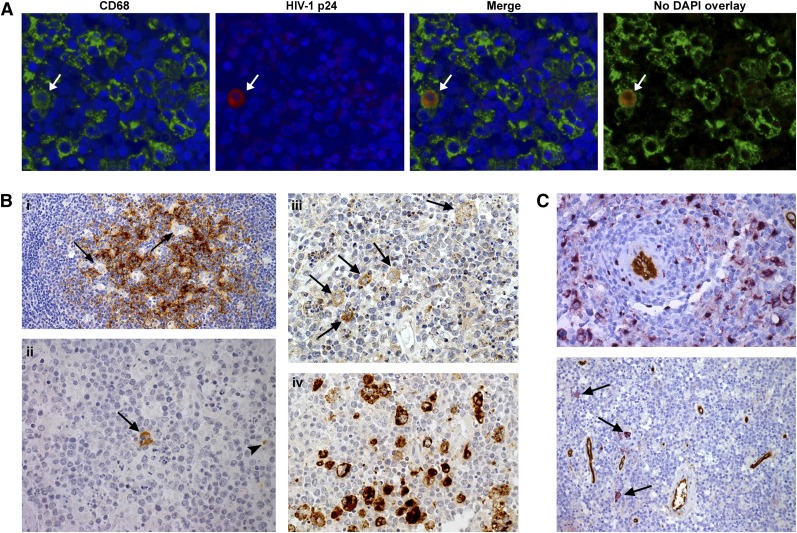
HIV-1 p24 antigen is a core component of HIV virions and its detection is characteristic of productive infection. HIV-1 p24 reactivity indicates active HIV replication with synthesis of virions within lymphoma tissue. Immunoperoxidase with anti-p24 was positive in 39% of specimens (Table 3). The number of positive cells was low, consisting of macrophages and reactive lymphocytes whereas lymphoma cells were negative. To ascertain the proportion of productively infected macrophages, we used immunofluorescence and demonstrated that HIV-1–producing macrophages represent <0.5% of the CD68+ population (Figure 3A). Such infected macrophages were especially seen in HAART-naive patients.
Table 3.
Summary of viral studies in AR-DLBCL specimens and clinicopathological features
| Viral expression | Total, no. (%) | Pre-HAART specimens, no. (%) | HAART-era specimens, no. (%) | Median CD4 cell count per mm3 (range) | Immunoblastic/plasmablastic morphology, no (%) |
|---|---|---|---|---|---|
| Viral status* | |||||
| HIV1 p24-positive | 16/41 (39) | 4/12 (33.3) | 12/29 (41.4) | 69.5 (10-840) | 7/16 (43.75) |
| EBER-positive | 23/41 (56.1) | 11/12 (91.7) | 12/29 (41.4) | 79 (10-840) | 21/23 (91.3) |
| LANA1-positive | 2/41 (4.8)† | 1/2 | 1/2 | 51 (19-83) | 2/2 |
| Viral profiles* | |||||
| EBER+, LMP1+, p24− | 11/41 (26.8) | 6/11 (54.5) | 5/11 (45.5) | 104.5 (20-380) | |
| EBER+, LMP1−, p24− | 6/41 (14.6) | 2/6 (33.3) | 4/6 (66.7) | 36.5 (25-300) | |
| EBER+, LMP1+, p24+ | 5/41 (12.2) | 3/5 (60) | 2/5 (40) | 64 (10-100) | |
| EBER+, LMP1−, p24+ | 1/41 (2.4) | 0/1 | 1/1 | 840 | |
| EBER−, LMP1−, p24+ | 10/41 (24.4) | 1/10 (10) | 9/10 (90) | 99.5 (12-228) | |
| EBER−, LMP1−, p24− | 8/41 (19.5) | 0/8 | 8/8 (100) | 280 (25-440) |
EBER, LANA1, and LMP1 were expressed by tumor cells; p24 was expressed by reactive lymphocytes and macrophages of the microenvironment.
The 2 cases positive for the nucleoprotein LANA1 of HHV-8 (also known as Kaposi sarcoma-associated herpes virus) had a history of Kaposi sarcoma. Both were EBER+ and one was also p24+. Among EBER+ tumors (23 of 41), cases with contemporaneous LMP1 expression (16 of 23), indicative of type II or III EBV persistence, included 15 (94%) immunoblastic and 1 (6%) plasmablastic lymphoma. Morphology differed in EBV+, LMP1− tumors characteristic of type I latency (7 of 23). In these cases, we noticed 2 (29%) immunoblastic, 3 (42%) plasmablastic, and 2 (29%) centroblastic lymphomas. These findings could suggest a mechanism for EBV latent gene products in cell morphology.
Figure 3.
Identification of HIV-1 in a positive specimen. (A) Double immunofluorescence using fluorescein isothiocyanate-conjugated antibody to CD68 in association with HIV-1 p24 (labeled with Texas Red). HIV-1–producing macrophages showing characteristic mixed (orange) fluorescence (white arrow) account for a small percentage of the total CD68+ cell population (<0.5%). Slides were scanned and analyzed with an Olympus BX61 fluorescent microscope. (B) Control tissue (persistent generalized lymphadenopathy) stained with p24 immunoperoxidase (i). The bulk of p24 is associated with follicular dendritic cell processes whereas the tingible-body macrophages (arrows) are negative (original magnification, ×100). In AR-DLBCL tissue (ii), few cells react with p24. The arrow indicates an infected macrophage and the arrowhead points to a lymphocyte (original magnification, ×400). Staining of the same AR-DLBCL biopsy for the HIV-1 regulatory tat protein (iii) revealed a higher number of infected macrophages (original magnification, ×400). (iv) The total macrophage population in this case is highlighted by the marker CD68. (C) Double staining using VIP (purple) for CD68 and DAB (brown) for CD34 (top) showing a ring of perivascular macrophages (original magnification, ×400). On double staining, p24 purple and CD34 brown (bottom), HIV-1–expressing macrophages (arrows) are not specifically seen perivascularly (original magnification, ×200). Images were obtained with a Leica DM2500 microscope.
The HIV-1 tat protein plays a central role in HIV-1 regulation by promoting the transcription of genes encoding structural proteins such as p24. Tat released from infected cells is notable for its ability to circulate in blood or to pass through cell membranes and cause deregulation of intracellular pathways in uninfected cells including endothelial cells and B cells.22 HIV-1, through its regulatory protein tat, has mechanistically been implicated in the pathogenesis of AR-DLBCL.23 Immunohistochemical analysis for tat protein revealed that although its cellular distribution paralleled the p24 results, the tat-positive macrophages exceeded the number of productively infected (p24-positive) cells (Figure 3B). Thus, HIV-1–expressing AR-DLBCL contains a mosaic of infected and uninfected macrophages. Endothelial or lymphoma cells were tat-negative. Unlike previous reports of perivascular localization of p24-positive macrophages,24 this localization was not observed in our study (Figure 3C).
Viral profiles and the biological basis of lymphocytic infiltration
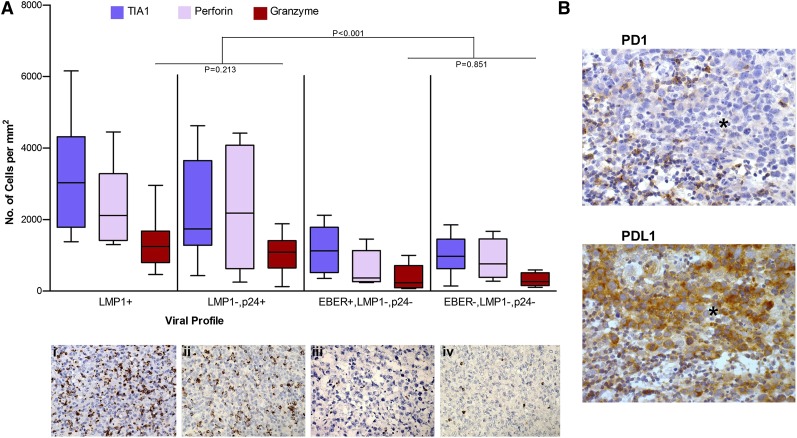
Profiling the viral expression within AR-DLBCL, tissue was pursued through staining for EBER and LMP1 for EBV, LANA1 for HHV-8, and p24 for HIV-1 infection. Our results on HIV-1 and co-infecting viruses together with clinical data are presented in Table 3. Co-infection with EBV or HHV-8 affected 60% of AR-DLBCL patients whereas ∼20% were negative for EBV, HHV-8, and HIV-1 p24. EBV-positive cases included 30% type I and 70% type II/III latency. These results may have implications for pathogenesis because co-infection with gammaherpesviruses is a major cofactor for the development of ARL. When we plotted the viral profiles against the amount of tumor-infiltrating lymphocytes in AR-DLBCL, we observed that biopsies positive for LMP1 and/or p24 contained a markedly higher number of CTL than cases negative for the 2 viral antigens. Characteristically, the number of granzyme-positive CTL was lower in cases without LMP1 and p24 expression (P < .001). CTL infiltration of EBER-positive, LMP1-negative, and p24-negative specimens did not differ from the pattern seen in EBER-negative, p24-negative specimens (Figure 4A). Thus, the cytotoxic cell infiltration of AR-DLBCL is dependent on the presence of LMP1 or p24 viral antigens.
Figure 4.
The infiltration of AR-DLBCL by CTLs is determined by the viral profile of the tumor. (A) The number of cytotoxic cells against the viral profiles. Cases with viral antigen expression (LMP1 and/or p24) show a substantially higher infiltration by cytotoxic cells than those lacking viral antigens. The P values correspond to activated (granzyme-positive) CTL. Immunohistochemical analysis for granzyme of 4 representative biopsy specimens with different viral profiles: (i) LMP1+ AR-DLBCL, (ii) LMP1−, p24+ AR-DLBCL, (iii) EBER+, LMP1−, p24−, and (iv) EBER−, LMP1−, p24− AR-DLBCL. (B) Additional immunosuppressive pathways in AR-DLBCL: PD1-PDL1 interaction. PD1-positive reactive lymphocytes are adjacent to, but also present within, the tumor whereas tumor cells demonstrate strong expression of the PD1 ligand, PDL1; *infiltrating sheets of tumor. Images were obtained with a Leica DM2500 microscope (original magnification, ×400), Leica DFC320 camera.
Immunoexhaustion and immunosenescence
PD1, an immune-checkpoint receptor, and its ligand PDL1 mediate a key inhibitory pathway called immunoexhaustion on activated T cells characterized by diminished cytotoxicity, decreased cytokine production, and increased apoptosis. T-cell exhaustion is present in chronic viral infections, where it has a negative impact on viral clearance, and in various cancers, where it is involved in tumor-mediated immune evasion.25,26 On immunohistochemical analysis, we identified PDL1-positive tumor cells in 28% and 26.8% of HIV-infected and uninfected cases, respectively. In AR-DLBCL, we noted a lower frequency of PD1-expressing T cells than in uninfected samples, consistent with the loss of CD4+ T cells entailing HIV-1 infection. No correlation was found between PDL1 or PD1 expression and CD4 cell count, numbers of tumor-infiltrating cytotoxic T cells, AIDS period, histology, or viral profile. Figure 4B shows an example of PD1-PDL1 interaction. The expression of CD57 on T lymphocytes reflects the proportion of cells with an inability to proliferate.27 In our study, levels of CD57 and C57:CD3 ratios did not differ between AR-DLBCL and sporadic DLBCL (P = .336 and P = .774, respectively).
Discussion
Our data show that features of HIV infection are echoed on the tumor microenvironment. An important biologic characteristic of AR-DLBCL is the depletion of CD4+ and FOXP3+ infiltrating T cells. Although the loss of cells was attenuated in the HAART era, their number remained significantly lower than in sporadic DLBCL. Several patients had not started HAART at the time of diagnosis as the lymphoma was their presenting illness. Two factors may account for the weak correlation between blood and tissue CD4+ T cells. First, the cancer-host immunologic interaction is complex, especially in the case of AIDS-defining cancer. The CD4 count is not an unbiased measure of the degree of immunodeficiency. What is more accurate than blood CD4 kinetics in predicting AIDS is the decay of tissue lymphocytes and the involution of lymphoid organs.28 Second, blood lymphocytes represent only 2% of the total lymphocyte pool and their composition differs from that within the organs during progressive HIV disease.29
There is considerable evidence to indicate that initiation of angiogenesis, the so-called angiogenic switch, is mediated by factors released by the tumor and often by macrophages attracted to it. In this study, we found that AR-DLBCL is characterized by markedly higher angiogenic activity compared with sporadic DLBCL. Our data show that neovascularization is tumor-driven and linked to immunoblastic features and the EBV status of tumor. Accumulating data from experimental studies provide helpful groundwork for the biological link between EBV infection and the process of new-vessel development. The latent membrane protein LMP1 is known for its ability to cause transformation, proliferation, and immortalization of B cells through tumor necrosis factor receptor-associated factor-dependent activation of nuclear factor κB and Akt protein kinase pathways.30 Signaling by LMP1 can alternatively upregulate HIF1 through Siah proteins resulting in vascular endothelial growth factor (VEGF) production and in this way promote angiogenesis.31 In cells undergoing lytic infection, a lytic viral gene has also been recognized as angiogenesis activator.32 Thus, induction of angiogenesis by EBV illustrates the important principle that distinct traits of neoplastic disease can be coregulated by the same transforming agent.33
HIV-1 may also be involved in angiogenesis. Supporting this concept is an experiment made in mice. The HIV-1 tat gene introduced into the mouse germline as well as injections of tat protein led to the formation of cutaneous vascular lesions characterized by capillary proliferation.34
Our preliminary data provide new perspectives for AR-DLBCL. Identifying the pathophysiology of angiogenesis in this disease could lead to improvements in our knowledge of the factors that control the angiogenic switch and enable these factors to be modified. Although our findings are consistent with tumor-driven angiogenesis, additional studies will be needed to define the relative roles of EBV, HIV-1, and possibly their synergism. What has practical implications is that the increased angiogenesis in AR-DLBCL represents an attractive target that could be exploited therapeutically. The monoclonal antibody to VEGF, bevacizumab, is currently being tested in clinical trials involving sporadic DLBCL and HIV-associated Kaposi sarcoma.8,35 Our observations suggest that angiogenesis inhibitors may be a promising new approach for AR-DLBCL, particularly EBV-positive immunoblastic/plasmablastic lymphoma which is characterized by marked angiogenesis and has an unfavorable outcome with standard treatment.6 Furthermore, HIV-protease inhibitors can directly affect angiogenesis due to inhibition of matrix metalloprotease activity.36 HAART containing a protease inhibitor may theoretically benefit the patient twofold by controlling HIV replication and tat and by limiting angiogenesis.
Another characteristic feature of AR-DLBCL was infiltration by CD8+ T cells. Given the central role of viral infection in AR-DLBCL, we looked for antiviral footprints by plotting the number of CTL against the viral profile in biopsy. Our analysis revealed that the frequency of CTL was prominently higher among LMP1 and/or p24-expressing cases. These results suggest that the cytotoxic response in AR-DLBCL is causally associated with the presence of viral antigens either on tumor cells (LMP1) or in the milieu (p24). We also observed that the granzyme-positive CTL were substantially higher in AR-DLBCL than in sporadic DLBCL. Thus, the picture that emerges illustrates viral antigen–expressing DLBCL as more likely to raise a cytotoxic response than viral antigen–negative or nonviral cases. Since a major function of granzymes is the maintenance of immunosurveillance,16 our data support the idea that surveillance of infection-linked cancer normally depends on reducing viral burden through killing virus-infected cells. Our findings might be also be taken as an argument that HIV patients, despite their immunosuppression, still have residual capability for immunologic defense against viral cancer mounted by CTL.
What are the implications of these observations for immunosurveillance? In viral antigen−positive AR-DLBCL, judging from the insufficiency of CTL to kill infected cells, pathways that impede cytotoxicity must occur. A recent study in mice pointed to the strategic role of CD4+ T cells in eliminating LMP1-positive cells and in keeping LMP1-driven lymphomas in check.37 In fact, CD4+ T cells had superior in vivo cytolytic activity against LMP1-transformed cells. The killing activity developed by CD4+ T cells is likely mediated by class II–dependent degranulation of granzymes.38 Extrapolating our findings on the composition of microenvironment and taking into account that both CD4+ and CD8+ T cells are necessary for cytotoxicity, the lack of LMP1-reactive CD4+ T cells could be the Achilles’ heel of the tumor-killing response against LMP1-positive AR-DLBCL. On the basis of this hypothesis, substantial increments in CD4+ T cells, through early initiation of HAART, should improve immunosurveillance and reduce the incidence of this type of AR-DLBCL. As such, AR-DLBCL expressing viral antigens is more likely to have an antitumor benefit after improvement of immunosurveillance. AR-DLBCL without viral antigens, like most sporadic DLBCL, is characterized by a low percentage of granzyme-positive CTL and thus this type of AR-DLBCL is best viewed as able to hide from immunosurveillance. After surveying additional immunosuppressive mechanisms, we noted PDL1 expression in 28% of AR-DLBCL. The significance of PD1-PDL1 in the immunotherapy of DLBCL remains to be defined, but this may have a therapeutic role. Preliminary evidence of clinical activity in various cancers suggest that immune-checkpoint-pathway inhibitors such as anti-PDL1 antibody may enhance T-cell responses and mediate antitumor activity.39
As mentioned, our data provide a model in which granzyme production by CTL within AR-DLBCL suggests that these cells are activated against viral antigen. Nonetheless, our work is subject to the limitations of studies in fixed specimens. Additional studies in fresh tissue will be needed to characterize the function of tumor-infiltrating CTL and confirm their specificity.
Studying tumor macrophages revealed no differences in their numbers between HIV-infected and uninfected patients. In addition, we did not find between-group difference in the 2 AIDS periods and we did not observe a correlation between macrophages and survival. However, these observations should be interpreted with caution because the propagating HIV-1 within macrophages in combination with the chronic inflammation, systemic activation, and opportunistic infections could cause dynamic variations in macrophages. As found in the analysis of HIV-infected macrophages, productive infection demonstrated by p24 protein occurred in rare cells but a higher number expressed the tat protein. Such macrophages were found in 39% of samples, typically from HAART-naive patients.
Our aim was to examine the basic characteristics of AR-DLBCL microenvironment. Obtaining a clear picture of the microenvironment and assembling this information into a coherent pathophysiological chain should inform mechanism-based strategies against immunodeficiency-linked tumors.
Supplementary Material
Acknowledgments
The authors thank Dr Fotios Panitsas for help on statistical results.
This work was supported by program grants P01 CA81538 (J.G.G.) from the National Cancer Institute at the National Institutes of Health and C1574/A6806 (J.G.G.) from Cancer Research UK.
K.L. has a Postgraduate Scholarship from the Hellenic Society of Hematology Foundation.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.L. designed and performed experiments, analyzed results, and wrote the manuscript; J.G.G. designed the research, supervised the study, and wrote the manuscript; A.C. designed and performed experiments and analyzed results; M.C. and A.M.L. provided expert histopathology review; S.M. provided clinical information; A.O. performed experiments and contributed to research; and R.C. and P.G. contributed to research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John G. Gribben, Barts Cancer Institute, Queen Mary University of London, John Vane Science Centre, Charterhouse Square, London, EC1M 6BQ, United Kingdom; e-mail: j.gribben@qmul.ac.uk.
References
- 1.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 3.Cheung MC, Pantanowitz L, Dezube BJ. AIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapy. Oncologist. 2005;10(6):412–426. doi: 10.1634/theoncologist.10-6-412. [DOI] [PubMed] [Google Scholar]
- 4.Tirelli U, Bernardi D. Impact of HAART on the clinical management of AIDS-related cancers. Eur J Cancer. 2001;37(10):1320–1324. doi: 10.1016/s0959-8049(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 6.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245–3255. doi: 10.1182/blood-2011-08-373738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stebbing J, Gazzard B, Douek DC. Where does HIV live? N Engl J Med. 2004;350(18):1872–1880. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 8.Lenz G, Wright G, Dave SS, et al. Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry AM, Cardesa-Salzmann TM, Meyer PN, et al. A new biologic prognostic model based on immunohistochemistry predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2012;120(11):2290–2296. doi: 10.1182/blood-2012-05-430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 11.Ventura RA, Martin-Subero JI, Jones M, et al. FISH analysis for the detection of lymphoma-associated chromosomal abnormalities in routine paraffin-embedded tissue. J Mol Diagn. 2006;8(2):141–151. doi: 10.2353/jmoldx.2006.050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 13.Clear AJ, Lee AM, Calaminici M, et al. Increased angiogenic sprouting in poor prognosis FL is associated with elevated numbers of CD163+ macrophages within the immediate sprouting microenvironment. Blood. 2010;115(24):5053–5056. doi: 10.1182/blood-2009-11-253260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 15.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 2001;97(3):744–751. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- 16.Podack ER. Execution and suicide: cytotoxic lymphocytes enforce Draconian laws through separate molecular pathways. Curr Opin Immunol. 1995;7(1):11–16. doi: 10.1016/0952-7915(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 17.Kamarashev J, Burg G, Mingari MC, Kempf W, Hofbauer G, Dummer R. Differential expression of cytotoxic molecules and killer cell inhibitory receptors in CD8+ and CD56+ cutaneous lymphomas. Am J Pathol. 2001;158(5):1593–1598. doi: 10.1016/S0002-9440(10)64114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med. 2005;143(4):265–273. doi: 10.7326/0003-4819-143-4-200508160-00007. [DOI] [PubMed] [Google Scholar]
- 19.Porcheray F, Samah B, Léone C, Dereuddre-Bosquet N, Gras G. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349(1):112–120. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Moses AV, Williams SE, Strussenberg JG, et al. HIV-1 induction of CD40 on endothelial cells promotes the outgrowth of AIDS-associated B-cell lymphomas. Nat Med. 1997;3(11):1242–1249. doi: 10.1038/nm1197-1242. [DOI] [PubMed] [Google Scholar]
- 21.Chirivi RG, Taraboletti G, Bani MR, et al. Human immunodeficiency virus-1 (HIV-1)-Tat protein promotes migration of acquired immunodeficiency syndrome-related lymphoma cells and enhances their adhesion to endothelial cells. Blood. 1999;94(5):1747–1754. [PubMed] [Google Scholar]
- 22.Li JC, Yim HC, Lau AS. Role of HIV-1 Tat in AIDS pathogenesis: its effects on cytokine dysregulation and contributions to the pathogenesis of opportunistic infection. AIDS. 2010;24(11):1609–1623. doi: 10.1097/QAD.0b013e32833ac6a0. [DOI] [PubMed] [Google Scholar]
- 23.De Falco G, Bellan C, Lazzi S, et al. Interaction between HIV-1 Tat and pRb2/p130: a possible mechanism in the pathogenesis of AIDS-related neoplasms. Oncogene. 2003;22(40):6214–6219. doi: 10.1038/sj.onc.1206637. [DOI] [PubMed] [Google Scholar]
- 24.Huysentruyt LC, McGrath MS. The role of macrophages in the development and progression of AIDS-related non-Hodgkin lymphoma. J Leukoc Biol. 2010;87(4):627–632. doi: 10.1189/jlb.0809564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 26.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg YJ, Zack PM, Leon EC, et al. Immunological and virological changes associated with decline in CD4/CD8 ratios in lymphoid organs of SIV-infected macaques. AIDS Res Hum Retroviruses. 1994;10(7):863–872. doi: 10.1089/aid.1994.10.863. [DOI] [PubMed] [Google Scholar]
- 29.Sopper S, Nierwetberg D, Halbach A, et al. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood. 2003;101(4):1213–1219. doi: 10.1182/blood-2002-06-1644. [DOI] [PubMed] [Google Scholar]
- 30.Shair KH, Bendt KM, Edwards RH, Bedford EC, Nielsen JN, Raab-Traub N. EBV latent membrane protein 1 activates Akt, NFkappaB, and Stat3 in B cell lymphomas. PLoS Pathog. 2007;3(11):e166. doi: 10.1371/journal.ppat.0030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo S, Seo SY, Yoshizaki T, et al. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 2006;66(20):9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- 32.Hong GK, Kumar P, Wang L, et al. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J Virol. 2005;79(22):13984–13992. doi: 10.1128/JVI.79.22.13984-13992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Ensoli B, Gendelman R, Markham P, et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371(6499):674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 35.Uldrick TS, Wyvill KM, Kumar P, et al. Phase II study of bevacizumab in patients with HIV-associated Kaposi’s sarcoma receiving antiretroviral therapy. J Clin Oncol. 2012;30(13):1476–1483. doi: 10.1200/JCO.2011.39.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. Lancet Oncol. 2003;4(9):537–547. doi: 10.1016/s1470-2045(03)01192-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Kracker S, Yasuda T, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148(4):739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.