Abstract
Background
Previously, we showed a mouse model (ACE8/8) of cardiac renin-angiotensin system (RAS) activation has a high rate of spontaneous ventricular tachycardia (VT) and sudden cardiac death (SCD) secondary to a reduction in connexin43 (Cx43) level. Angiotensin-II activation increases reactive oxygen species (ROS) production, and ACE8/8 mice show increased cardiac ROS. We sought to determine the source of ROS and if ROS played a role in the arrhythmogenesis.
Methods and Results
Wild-type and ACE8/8 mice with and without two weeks of treatment with L-NIO (nitric oxide synthase inhibitor), sepiapterin (precursor of tetrahydrobiopterin), MitoTEMPO (mitochondria-targeted antioxidant), TEMPOL (a general antioxidant), apocynin (NADPH oxidase inhibitor), allopurinol (xanthine oxidase inhibitor), and ACE8/8 crossed with P67 dominant negative mice to inhibit the NADPH oxidase were studied. Western blotting, detection of mitochondrial ROS by MitoSOX Red, electron microscopy, immunohistochemistry, fluorescent dye diffusion technique for functional assessment of Cx43, telemetry monitoring, and in-vivo electrophysiology studies were performed. Treatment with MitoTEMPO reduced SCD in ACE8/8 mice (from 74% to 18%, P<0.005), decreased spontaneous ventricular premature beats, decreased VT inducibility (from 90% to 17%, P<0.05), diminished elevated mitochondrial ROS to the control level, prevented structural damage to mitochondria, resulted in 2.6 fold increase in Cx43 level at the gap junctions, and corrected gap junction conduction. None of the other antioxidant therapies prevented VT and SCD in ACE8/8 mice.
Conclusions
Mitochondrial oxidative stress plays a central role in angiotensin II-induced gap junction remodeling and arrhythmia. Mitochondria-targeted antioxidants may be effective antiarrhythmic drugs in cases of RAS activation.
Keywords: sudden cardiac death, ventricular tachycardia, oxidative stress, mitochondria
Introduction
An increased level of angiotensin-II (AngII), as is found in heart failure, is associated with an increased risk of ventricular tachycardia (VT), and treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers reduces that risk.1 Investigating the mechanisms of AngII-induced arrhythmia may result in finding new antiarrhythmic targets. We created a mouse model of cardiac-restricted angiotensin converting enzyme (ACE) overexpression. We demonstrated that homozygous mice (ACE8/8) have a high rate of sudden cardiac death (SCD), with telemetry monitoring showing that approximately 80% of the SCD resulted from VT and less commonly severe bradycardia and conduction block, in the absence of any left ventricular (LV) structural or functional abnormality at the studied age.2 The VT and bradycardia were the result of c-Src tyrosine kinase activation, connexin43 (Cx43) reduction, and the impairment of gap junction conduction.2,3
AngII is known to increase reactive oxygen species (ROS) levels.4 Excess amounts of ROS have been implicated in the genesis of arrhythmia,2,5,6 and ROS is known to activate c-Src.7 Nevertheless, there is no clear proof that oxidative stress causes arrhythmia or of how oxidative stress might contribute to the arrhythmic substrate. Therefore, we sought to determine whether ROS mediated any of the Cx43 remodeling during renin-angiotensin system (RAS) activation and the principal source of cardiac ROS responsible for arrhythmic risk. Sources of ROS include the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that generally requires the p67 subunit for activity, xanthine oxidase, uncoupled nitric oxide synthase (NOS) in part because of tetrahydrobiopterin depletion, and mitochondria.8 We inhibited each source in turn using previously established methods9-15 and explored the effect on RAS-induced arrhythmogenesis.
Materials and Methods
The animal experiments were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Experimental Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee. All mice in this study were started on treatment at the age of 4 weeks, and they were studied for 2 weeks. In our previous studies of ACE8/8 mice, we did not detect any difference in the phenotypes based on the mouse sex.2,3 Therefore, we used both sexes for our experiments. A group of wild-type mice (n=10) with similar background to the ACE8/8 mice (C57BL), and the following groups of ACE8/8 mice were studied:
ACE8/8 mice untreated control (n=23).
ACE8/8 mice treated with 4′-hydroxy-3′methoxyacetophenone (apocynin; Sigma-Aldrich, St. Louis, MO) to inhibit the NADPH oxidase activity (1.5 mmol/L in drinking water for two weeks, n=8).13
ACE8/8 mice crossed with a P67 dominant negative (P67DN) mice to inhibit NADPH oxidase activity (n=10). P67 is an important subunit of NADPH oxidase.14
ACE8/8 mice treated with N5-(1-iminoethyl)-L-ornithine, dihydrochloride (L-NIO; Sigma-Aldrich) to inhibit nitric oxide synthase (NOS) (25mg/Kg/d intraperitoneal injections for two weeks, n=10). L-NIO is an inhibitor of all NOS subtypes.12
ACE8/8 mice treated with 2-amino-7,8-dihydro-6-(2S-hydroxy-1-oxopropyl)-4(1H)-pteridinone (sepiapterin; Sigma-Aldrich), a precursor of tetrahydrobiopterin, to prevent eNOS uncoupling without inhibition of NOS (5 mg/Kg/d intraperitoneal injections for two weeks, n=8).15
ACE8/8 mice treated with 1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (allopurinol) (Sigma-Aldrich) to inhibit xanthine oxidase (1 mmol/L in the drinking water for two weeks, n=10).11
ACE8/8 mice treated with 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (TEMPOL; Enzo Life Sciences), which is a general antioxidant and mimetic of superoxide dismutase (2 mmol/L in drinking water for two weeks, n=8).10
ACE8/8 mice treated with (2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO; Enzo Life Sciences, Plymouth Meeting, PA) to target mitochondrial superoxide (0.7 mg/Kg/d intraperitoneal injections for two weeks, n=17).9
In addition, a group of wild-type mice were treated with MitoTEMPO (0.7 mg/Kg/d intraperitoneal injections for two weeks, n=5) to evaluate for any possible harmful effects of treatment.
Survival Recording and Analysis
Survival of all treated and untreated groups were recorded every morning during the two weeks of treatment and/or observation. Survival was assessed by using Kaplan-Meier analysis and log rank tests.
Telemetry Monitoring
Please see supplemental methods.
Electrophysiology Study
For the electrophysiology studies, the control mice (n=5), ACE8/8 mice (n=10) and ACE8/8 mice treated with MitoTEMPO (n=6) were studied as previously described (see supplemental methods).2
Mitochondrial ROS Measurement by Confocal Microscopy
To measure mitochondrial ROS, the fluorescent probe MitoSOX Red was used as previously described.16 Cardiomyocytes were isolated from control, ACE8/8 or ACE8/8 mice treated with MitoTEMPO (n=3 for each group; see supplemental methods).
Mitochondrial ROS Measurement by Flow Cytometry
To quantify the mitochondrial ROS by flow cytometry, the measurements were carried out using Cyan ADP (Beckman Coulter, Brea, CA). Isolated cardiomyocytes from each group (n=3 animals for each group) were stained with 5 μM MitoSOX Red with a similar method as above (see supplemental methods).
Transmission Electron Microscopy
Control, ACE8/8 mice, and ACE8/8 mice treated with MitoTEMPO were studied (n=3 for each group; see supplemental methods).
Western Blot Analysis
The control, ACE8/8, and ACE8/8 treated with MitoTEMPO mice (n–5 for each group) were sacrificed, and their hearts were excised (see supplemental methods).
Immunohistochemistry
Control, ACE8/8, and ACE8/8 treated with MitoTEMPO mouse hearts (n=4 for each group) were fixed in 10% formalin. After which, 8-μm thick sections were blocked for 1h at room temperature and then were incubated with anti-Cx43 antibodies (Cell Signaling) overnight at 4°C at concentrations known to provide the best signal-to-noise ratio. This method has been used previously to quantify levels of collagen and Cx43 in cardiac tissue (see supplemental methods).2,17
Functional Assessment of Cx43
We used an established technique for measuring Cx43 function that involves fluorescent dye introduction and diffusion in intact heart muscle (see supplemental methods).
Statistical Analysis
The values are presented as the mean ± SEM. The values are presented as the mean ± SEM. The t test was used to evaluate the statistical significance between two groups for analysis of the mitochondrial ROS measurement, Western blot, immunohistochemistry, and electron microscopy results. One-way analysis of variance with posthoc Tukey honestly significant test were used to evaluate the statistical significance among the groups for the analysis of the dye diffusion test. The Fisher exact test for 2 × 2 tables was used for analysis of the VT inducibility by electrophysiology tests. A P value of < 0.05 was reported as statistically significant. The survival data were analyzed with the Kaplan-Meier method, and the P value was calculated with the log-rank test.
Results
Mitochondria-Targeted Antioxidant Therapy Prevented Sudden Cardiac Death and Inducibility of Ventricular Tachycardia
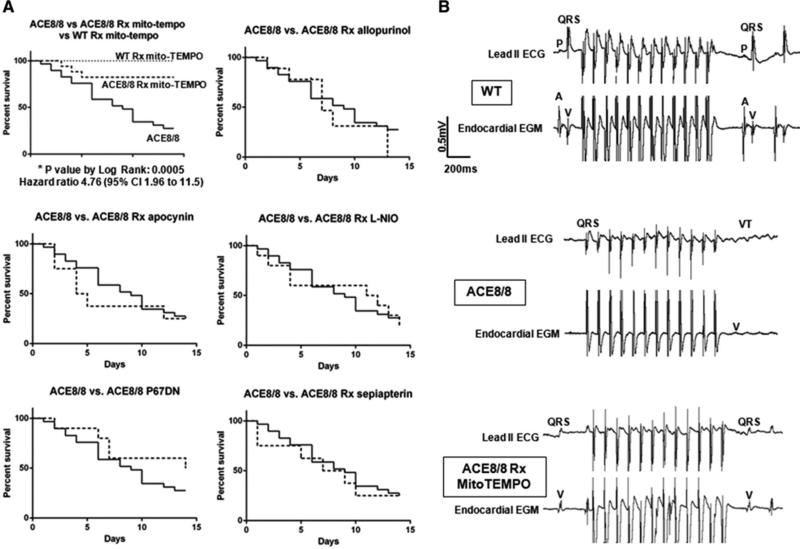
During two weeks of observation with various antioxidants (please see the Materials and Methods for details), only treatment with MitoTEMPO prevented SCD and improved survival in the ACE8/8 mice (from 26% to 82%; hazard ratio 4.8, 95% CI: 2.0 to 11.5; P<0.005). Although the NADPH oxidase, xanthine oxidase, and uncoupled NOS are potential sources of increased cardiac ROS with RAS activation, none of the other antioxidant therapies prevented SCD (Figure 1a). Treatment with TEMPOL, a general antioxidant that is similar to MitoTEMPO but it is not targeted to mitochondria, was not associated with improvement of survival free of sudden arrhythmic death (Supplemental Figure 1). Treatment of control mice with MitoTEMPO did not cause any death or gross abnormality in the treated mice. Telemetric monitoring of ACE8/8 (n=5) and ACE8/8 treated with MitoTEMPO (n=4) mice for two weeks revealed all mice who died did so from VT degenerating to VF. Mice treated with MitoTEMPO had a significantly reduced burden of premature ventricular beats (0.75 ± 0.2 vs. 4.4 ± 2.2 premature beats/min, P < 0.05) and only untreated mice showed nonsustained VT. Basic ECG parameters were comparable between groups (Supplemental Table 1).
Figure 1.
A mitochondrial antioxidant inhibits sudden cardiac death and ventricular arrhythmia inducibility. (a) RAS-activation mice were treated with the following antioxidants: apocynin, L-NIO, sepiapterin, allopurinol, TEMPOL, and MitoTEMPOL. A group of ACE8/8 mice were also crossed with P67DN mice. Kaplan-Meier survival analysis and log-rank tests show significant improvement in the survival free from sudden arrhythmic death only in the ACE8/8 mice that were treated with MitoTEMPO [Allopurinol: p=0.49, hazard ratio 0.75 (CI: 0.28 to 1.79); Apocynin: p=0.54, hazard ratio 0.77 (CI: 0.27 to 1.94); L-NIO: p=0.9024, hazard ratio 0.9526 (CI: 0.42 to 2.16); p67DN: p=0.22, hazard ratio 1.77 (CI: 0.74 to 4.01); Sepiapterin: p=0.67, hazard ratio 0.83 (CI: 0.31 to 2.10)]. MitoTEMPO had no effect on wild-type mice (WT). (b) Representative electrocardiograms (ECG lead II) and right ventricular electrograms (endocardial EGM) of WT, ACE8/8 and ACE8/8 mice treated with MitoTEMPO are shown. VT was induced in 90% of ACE8/8 mice (9 of 10) using a burst pacing protocol starting at 100 ms pacing cycle length (PCL) and decreasing to 30 ms PCL or 2:1 capture. Treatment with MitoTEMPO reduced VT inducibility in ACE8/8 mice to 17% (one of 6 mice) using the same above pacing protocol (P<0.05).
In the in-vivo electrophysiology studies, VT was induced in 90% (nine of 10) of ACE8/8 mice using a burst pacing protocol with a mean pacing cycle length (PCL) of 44 ms. The induced VTs in the ACE8/8 mice were primarily monomorphic (88%). VT inducibility in ACE8/8 mice was decreased from 90% to 17% (one of six) by MitoTEMPO treatment (P<0.05) (Figure 1b). VT could not be induced in control mice.
MitoTEMPO Treatment Reduced Mitochondrial Superoxide Levels
Quantification of mitochondrial ROS levels by the MitoSOX reduction and flow cytometry methods revealed a 1.5-fold increase in the mitochondrial superoxide level in the ACE8/8 mice compared to the control mice (p<0.05) (Figure 2a). MitoTEMPO treatment reduced mitochondrial ROS level to 1.1-fold of that in the control mice (P=0.45) (Figure 2a). Quantification of mitochondria by MitoTracker Green did not show any significant change between those groups (Figure 2b).
Figure 2.
Mitochondrial ROS is Increased in RAS Activation, (a) Mitochondrial ROS was measured using MitoSOX fluorescence. Representative confocal microscopy images show an increase in the mitochondrial superoxide level in ACE8/8 cardiomyocytes and suppression of that level with MitoTEMPO treatment. Flow cytometry analysis shows a 1.5 fold increase in the level of mitochondrial superoxide in ACE8/8 mice and MitoTEMPO decreased that level to normal. (b) MitoTracker Green was used to quantify mitochondria. There is no significant difference among the control, ACE8/8 and ACE8/8 treated with MitoTEMPO groups (n=10 for each group, P=0.85) in mitochondrial number.
MitoTEMPO Reversed Mitochondrial Damage in RAS Activation
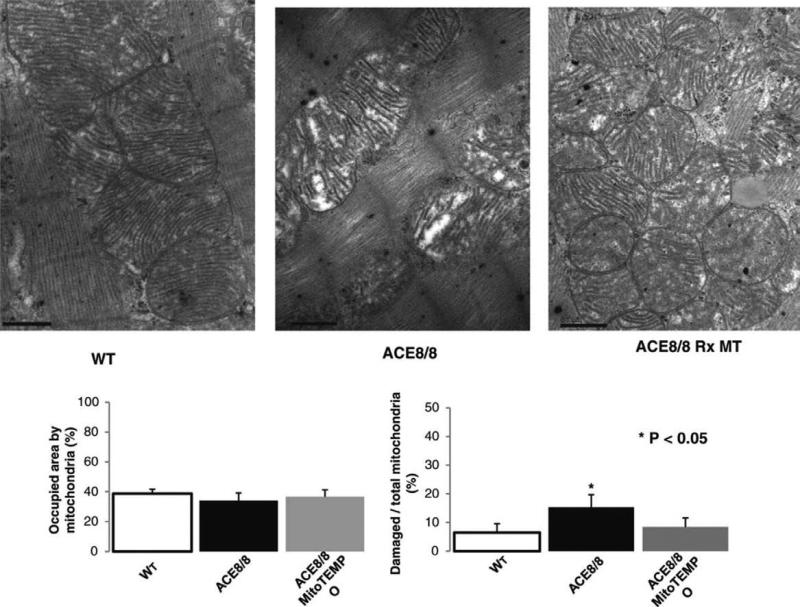
By electron microscopy, the percent of the cytoplasmic area occupied by mitochondria was not statistically different among the groups studied, consistent with mitochondria quantification with MitoTracker Green (Figure 3). Nevertheless, ACE8/8 mice showed significant damage to the mitochondria inner membrane and cisterna (Figure 3 and Supplemental Figure 2). The damaged area identified by the ratio of vacuous area within a mitochondrion to the whole mitochondrion was significantly higher in the ACE8/8 than in the control mice. This ratio was ameliorated by MitoTEMPO treatment (6.5 ± 3%, 15 ± 4%, and 8.5 ± 3% in the control, the ACE8/8, and the ACE8/8 mice treated with MitoTEMPO, respectively; P<0.05 for control compared to ACE8/8 mice). While mitochondria morphology was improved with two weeks of MitoTEMPO treatment, we did not study the time course or durability of the improvements in this study.
Figure 3.
RAS Activation was Associated with Mitochondrial Injury. Electron microscopy shows damage to the inner membrane and cisterna of mitochondria and vacuous areas within mitochondria areas with RAS activation that are prevented by MitoTEMPO treatment. RAS activation did not significantly change the percent area occupied by mitochondria compared with the control (38 ± 2%, 34 ± 5%, 36 ± 4% of cytoplasmic surface area, for control, ACE8/8, MitoTEMPO groups, respectively; P=0.16 comparing control vs. ACE8/8, and P=0.45 comparing ACE8/8 vs. MitoTEMPO), a finding consistent with the MitoTracker Green analysis.
Rarely could gap junctions be identified in untreated ACE8/8 cardiomyocytes, but gap junctions could be easily identified in the control and treated groups. Histological analysis and annexin V staining showed no differences in necrosis or apoptosis between treated and untreated groups accompanying these structural changes in mitochondria.
MitoTEMPO Increased Connexin43 Levels at the Gap Junctions
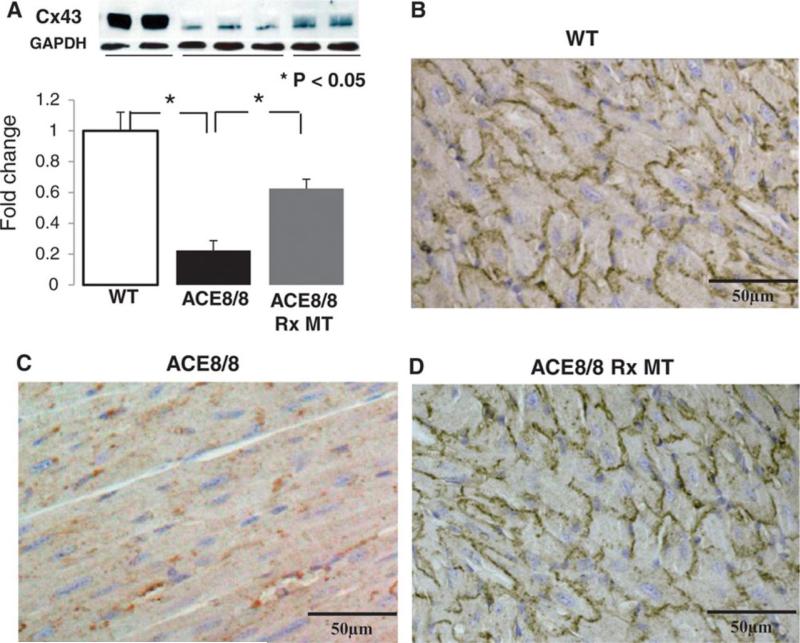
The total Cx43 level detected by Western blot was decreased in ACE8/8 mice to 24% of control (P<0.05), and MitoTEMPO treatment increased that to 62% of control (P<0.05) (Figure 4a). By immunohistochemistry, Cx43 level in ACE8/8 mice was decreased at intercalated disks to a comparable level measured by Western blot, and most of the remaining Cx43 in the untreated ACE8/8 mice was no longer located at identifiable intercalated disks (figure 4b). Cx43 increased significantly after MitoTEMPO treatment (p<0.05). Src is known to displace Cx43 from the intercalated disk,19 and the level of active c-Src, phospho-(Tyr416), was 32% higher in ACE8/8 than in control mouse hearts. Phospho-Src was reduced to that of control mice by MitoTEMPO treatment (P=0.29 compared to control) (Supplemental Figure 2).
Figure 4.
A Mitochondrial Antioxidant Recovers Cx43 in RAS-Activation Mice. (a) MitoTEMPO increases the total Cx43 level in ACE8/8 mice from 24% to 62% of the Cx43 level in the control mice (P<0.05). (b) Immunohistochemistry staining for Cx43 confirms the increase in Cx43 level in ACE8/8 mice by MitoTEMPO treatment.
MitoTEMPO Increased Gap Junction Conduction to the Control Level
To determine whether changes in Cx43 levels resulted in functional changes in gap junction conduction at the whole heart level, an established method of fluorescent dye diffusion was used (Figure 5). The predominant effect of MitoTEMPO on improving the dye diffusion longitudinally was consistent with the improvement of Cx43 level at the gap junctions in immunostaining experiments. The gap junction dye diffusion in the longitudinal direction was reduced in untreated ACE8/8 mice to 62% of that in the control mice (P<0.05). MitoTEMPO treatment returned the gap junction conduction to the normal range (P=0.97 compared to control).
Figure 5.
Cx43 Function is Improved with a Mitochondrial Anti-Oxidant. Cx43 functional assessment by the fluorescent dye diffusion technique reveals an increase in dye spread in ACE8/8 mouse hearts with MitoTEMPO treatment.
Discussion
Cx43 is the major structural protein of ventricular gap junctions, and a significant decrease in Cx43 causes sudden arrhy mic death.20 In this study, we showed that cardiac RAS activation, as occurs in heart failure,1,21 was associated with a significant reduction in Cx43. This range of reduction in Cx43 is known to be arrhythmogenic.22 These experiments establish that AngII-mediated ROS plays a role in ventricular arrhythmogenesis, that the mitochondria are the principle source of ROS leading to the arrhythmic substrate, and ROS is arrhythmogenic, at least in part, by altering Cx43 probably by ROS activation of c-Src.23,24
Although ROS have been implicated in the genesis of arrhythmia,25-29 translation of those findings to clinical studies using general ROS scavengers such as vitamin E and C have not produced impressive results.30 In our study, only a mitochondria-targeted antioxidant was able to prevent arrhythmia. Targeting other known cardiac sources of ROS or using a general antioxidant were ineffective despite dosages and routes of administration that have been shown to be effective in inhibition of the targeted source of ROS production.9-15 This result, particularly the therapeutic difference between TEMPOL and MitoTEMPO treatments, suggests that AngII-mediated ROS production is highly compartmentalized within mitochondria in cardiomyocytes.
It has been recently shown that AngII receptors exist on the mitochondrial inner membrane,31 and AngII may affect directly mitochondrial ROS production. In addition, an isoform of the NADPH oxidase (NOX4) exists in mitochondria,32,33 and AngII is known to activate NADPH oxidase.34,35 While our experiments do not suggest a role for the conventional NADPH oxidase, NOX4 does not require the P67 subunit for its activation,36 and apocynin may not effectively inhibit mitochondrial NOX4-dependent ROS production. Therefore, it is possible that this system could be involved in what appears to be AngII signaling directly to mitochondria, possibly through a ROS-induced-ROS mechanism.37
Our study does not preclude the possibility of other sources of ROS contributing to arrhythmogenesis in other cardiac pathological states. The RAS activation model used leaves open the possibility that inhibition of other sources of ROS could be effective in more complicated disease states. Moreover, it has been shown that perfusion of the whole heart or isolated cardiomyocytes with H2O2 is arrhythmogenic, which highlights the importance of the amount of ROS production in arrhythmogenesis, independent of the source of ROS.26 Consistent with others,5 we found that more than 30% of the cardiomyocyte area was occupied by mitochondria, and these mitochondria were producing 1.5 times higher superoxide in RAS activation mice than in control mice. Therefore, our results may simply be a function of the relative amounts of the enzymatic ROS sources in a cardiomyocyte. Similar findings of the importance of mitochondria as a source of ROS and accompanying mitochondrial damage were recently reported in other cardiac pathologies such as heart failure, a RAS activation state.5,38,39
These results may have clinical implications in patients with heart failure because AngII and ROS are elevated in that condition.1,21,40-43 Cx43 is reduced in heart failure, and sudden death is increased.44,45 Our findings collectively can be explained by a signaling cascade where cardiac RAS activation increases mitochondrial ROS production and mitochondrial injury, activates c-Src, reduces Cx43 at intercalated disks through competition with activated c-Src, reduces gap junction function, and increases ventricular arrhythmias (Figure 6). This proposed signaling cascade could explain why angiotensin converting enzyme inhibitors and AngII receptor blockers decrease sudden death.1,41 In addition, these results may have clinical implications in pathological conditions with elevated levels of ROS and c-Src activation exclusive of AngII, for example, in cardiac ischemia and in ischemia-reperfusion state.19,46
Figure 6.
Proposed Signaling Cascade of RAS-Induced Arrhythmogenesis. Activation of AngII significantly increases mitochondrial ROS production which in turn activates c-Src and results in Cx43 reduction at the gap junctions. Impaired gap junction conduction provides substrate for ventricular arrhythmia and sudden arrhythmic death.
Limitations
Cardiac restricted elevation of AngII in this model without hypertension and systolic dysfunction allowed investigation of the direct arrhythmogenesis effects of AngII in the heart. Although it is not generally expected, the results may vary in systemic elevation of AngII. It is also possible that MitoTEMPO exerted part of its antiarrhythmic effects by mechanisms other than c-Src activation and Cx43 remodeling. On the other hand, the lack of ventricular fibrosis, normal cardiac sodium current, and an unchanged ventricular effective refractory period in the ACE8/8 mice at the age they were studied support a major role for mitochondrial ROS in RAS-mediated Cx43 remodeling.2,3,47,48 While the major effect of MitoTEMPO treatment appeared to be to increase the amount of Cx43, MitoTEMPO treatment also appeared to increased Cx43 phosphorylation, which may help explain the improvement noted in connexon function. The reason for the early mortality in the MitoTEMPO treated mice is unknown, but it is possible that antioxidant treatment takes several days to reach maximum effect. Since treatments were not continued for greater than two weeks, it is unclear whether MitoTEMPO treatment prevented sudden death or simply delayed it.
Conclusions
In summary, we found that RAS activation resulted in mitochondrial injury, mitochondrial ROS production, a reduction in Cx43, and increased arrhythmic risk. These changes were ameliorated by a mitochondria-targeted antioxidant but not agents targeted to other sources of cardiac oxidation or a general antioxidant. These results establish that ROS can be arrhythmogenic and elucidate a possible mechanism whereby ROS can cause arrhythmia.
Supplementary Material
Acknowledgments
Funding Sources: RO1 HL1024025, T32 HL072742, P01 HL058000, R01 HL106592, a VA MERIT grant, and an American Heart Association Midwest Affiliate Postdoctoral Fellowship # AHA10POST4450037.
Footnotes
Conflict of Interest Disclosure: Dr. Dudley has submitted patent entitled, “Mitochondrial anti oxidants for prevention of sudden death by raising connexin43 levels” based on this work.
References
- 1.Teo KK, Mitchell LB, Pogue J, Bosch J, Dagenais G, Yusuf S. Effect of ramipril in reducing sudden deaths and nonfatal cardiac arrests in high-risk individuals without heart failure or left ventricular dysfunction. Circulation. 2004;110:1413–1417. doi: 10.1161/01.CIR.0000141729.01918.D4. [DOI] [PubMed] [Google Scholar]
- 2.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol. 2011;58:2332–2339. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iravanian S, Sovari AA, Lardin HA, Liu H, Xiao HD, Dolmatova E, Jiao Z, Harris BS, Witham EA, Gourdie RG, Duffy HS, Bernstein KE, Dudley SC., Jr Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med (Berl) 2011;89:677–687. doi: 10.1007/s00109-011-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DA, O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC., Jr Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–463. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab. 2007;293:E355–E363. doi: 10.1152/ajpendo.00632.2006. [DOI] [PubMed] [Google Scholar]
- 8.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med. 2011;50:777–793. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 10.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mendez I, Garrett MC, Adams AG, Leto TL. Role of p67-phox SH3 domains in assembly of the NADPH oxidase system. J Biol Chem. 1994;269:16326–16332. [PubMed] [Google Scholar]
- 15.Shen RS, Alam A, Zhang YX. Inhibition of GTP cyclohydrolase I by pterins. Biochim Biophys Acta. 1988;965:9–15. doi: 10.1016/0304-4165(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Liu H, Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107:967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita N, Lee JH, Bapat A, Fishbein MC, Mandel WJ, Chen PS, Weiss JN, Karagueuzian HS. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am J Physiol Heart Circ Physiol. 2011;301:H180–H191. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 19.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Norstrand DW, Asimaki A, Rubinos C, Dolmatova E, Srinivas M, Tester DJ, Saffitz JE, Duffy HS, Ackerman MJ. Connexin43 mutation causes heterogeneous gap junction loss and sudden infant death. Circulation. 2012;125:474–481. doi: 10.1161/CIRCULATIONAHA.111.057224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, Sanz G. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 22.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci USA. 2009;106:2983–2988. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol. 2009;297:H1594–H1605. doi: 10.1152/ajpheart.00579.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, Rengo F, Ambrosio G. Age-related decrease in cardiac tolerance to oxidative stress. J Mol Cell Cardiol. 1999;31:227–236. doi: 10.1006/jmcc.1998.0862. [DOI] [PubMed] [Google Scholar]
- 29.Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol. 2002;39:148–156. doi: 10.1016/s0735-1097(01)01709-0. [DOI] [PubMed] [Google Scholar]
- 30.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 35.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 36.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 37.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, van Gilst WH, Voors AA. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–372. doi: 10.1016/j.ijcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 42.Canton M, Menazza S, Sheeran FL, Polverino de LP, Di LF, Pepe S. Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol. 2011;57:300–309. doi: 10.1016/j.jacc.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 43.Banfi C, Brioschi M, Barcella S, Veglia F, Biglioli P, Tremoli E, Agostoni P. Oxidized proteins in plasma of patients with heart failure: role in endothelial damage. Eur J Heart Fail. 2008;10:244–251. doi: 10.1016/j.ejheart.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaprielian RR, Gunning M, Dupont E, Sheppard MN, Rothery SM, Underwood R, Pennell DJ, Fox K, Pepper J, Poole-Wilson PA, Severs NJ. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–660. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 46.Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J Cardiovasc Pharmacol Ther. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- 47.Kasi VS, Xiao HD, Shang LL, Iravanian S, Langberg J, Witham EA, Jiao Z, Gallego CJ, Bernstein KE, Dudley SC., Jr Cardiac-restricted angiotensin-converting enzyme overexpression causes conduction defects and connexin dysregulation. Am J Physiol Heart Circ Physiol. 2007;293:H182–H192. doi: 10.1152/ajpheart.00684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr., Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.