Abstract
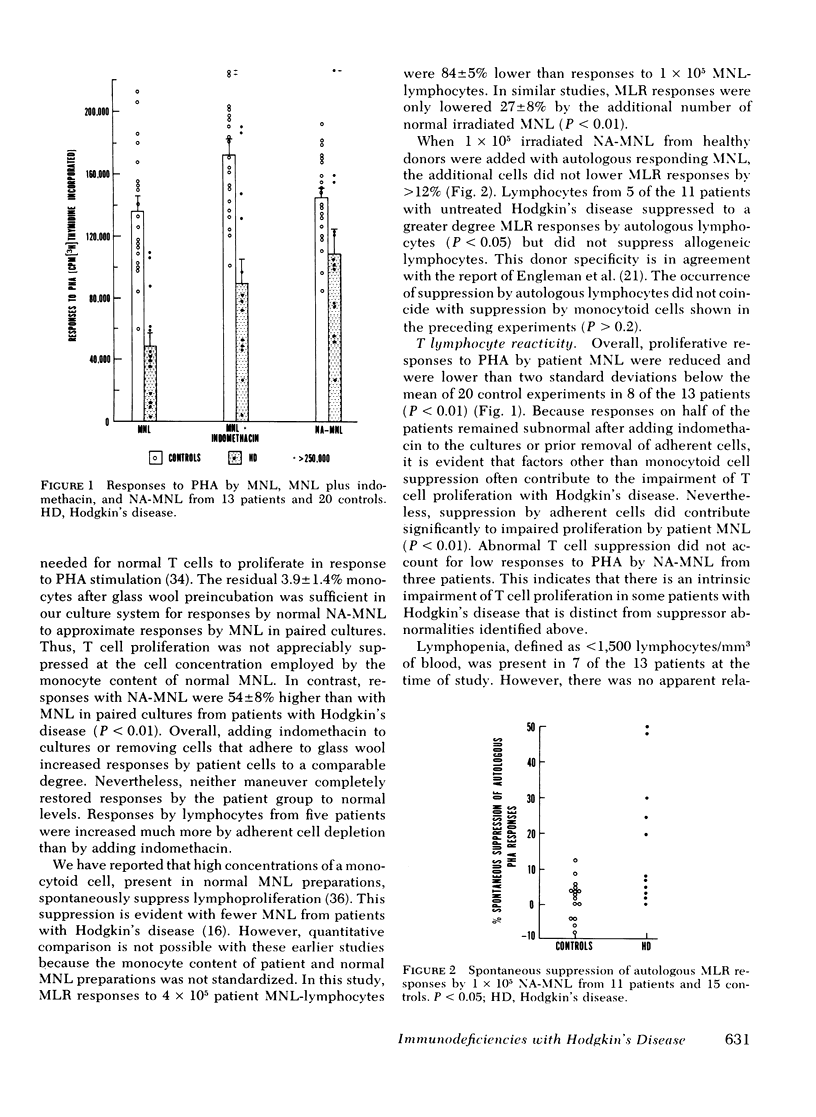
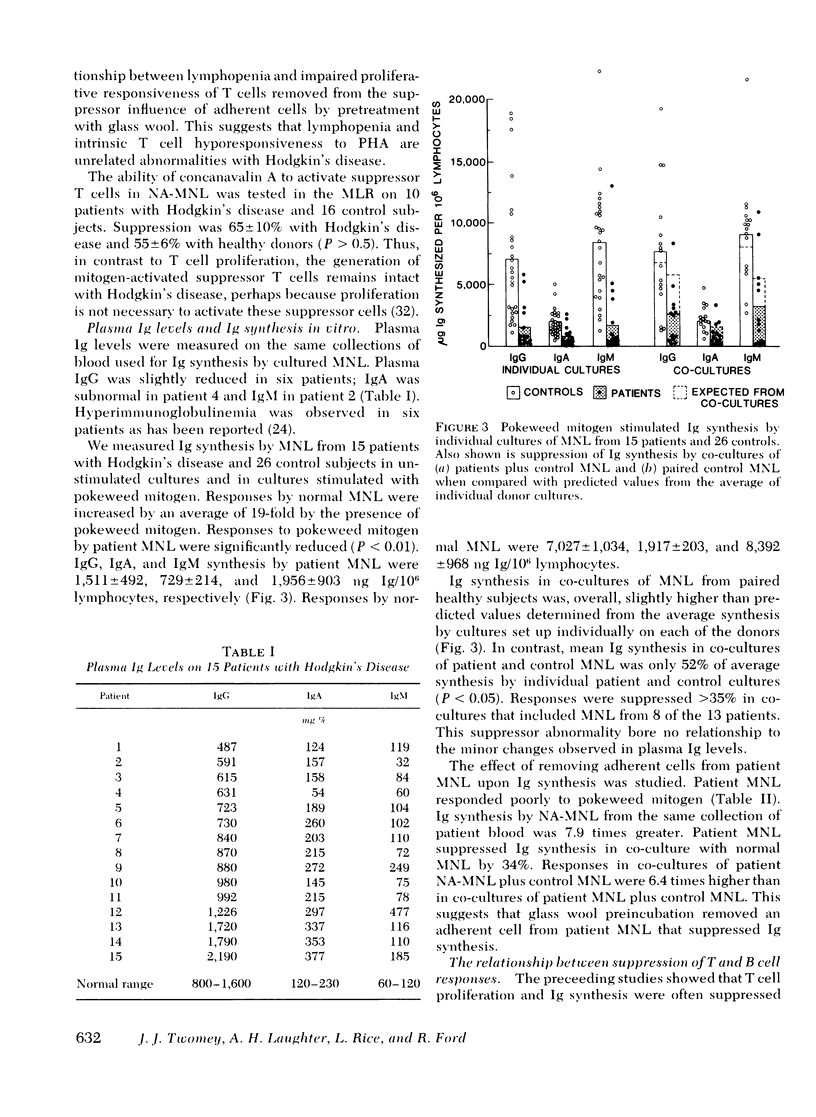
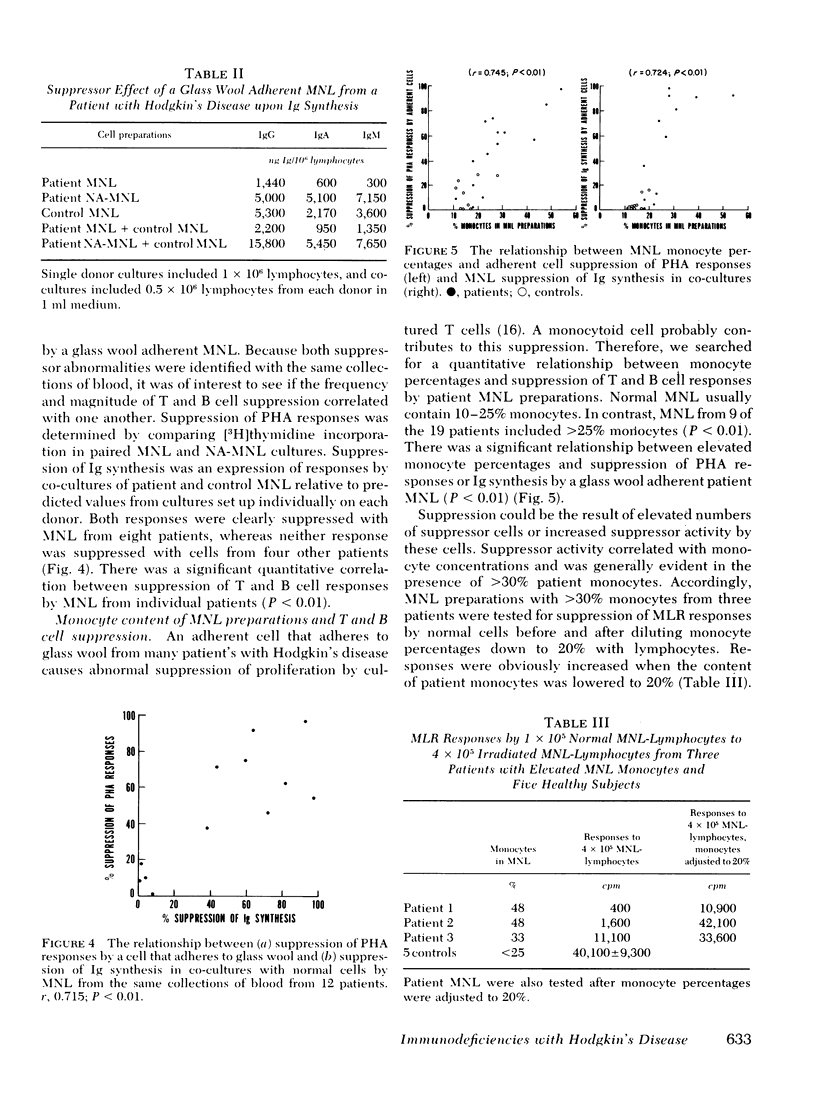
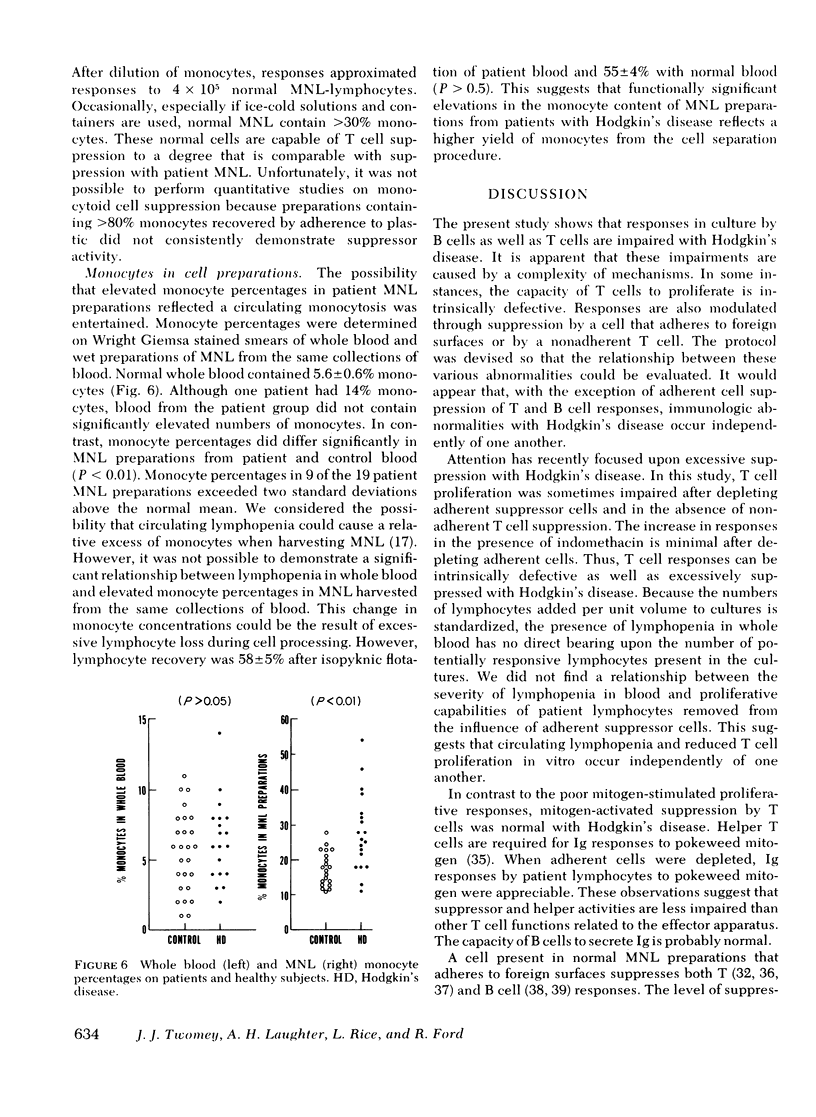
The role of six suppressor mechanisms upon T and B cell responses was studied on 17 untreated patients with Hodgkin's disease. Proliferative hyporesponsiveness to mitogen was greatly impaired in 8 of the 13 patients. 10 of these patients had an excessive degree of suppression by cells that adhered to foreign surfaces. Suppression by adherent cells correlated with impairment of proliferative responses and, in some instances, suppression was largely inhibited with indomethacin. Likewise, adherent cells suppressed immunoglobulin synthesis. A correlation was evident between suppression of T and B cell responses by adherent mononuclear leukocytes from individual patients. This suppression coincided with elevated percentages of monocytes in the patient mononuclear cell preparations. This excess of monocytes was not the result of a circulating monocytosis. The monocyte excess may have been acquired during isopyknic cell separation. A second form of suppression was observed in 5 of the 11 patients affected by a lymphocyte that neither adhered to glass wool nor required preactivation. It did not inhibit allogeneic lymphocytes, which contrasts with the suppressor abnormality of monocytoid cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carbone P. P., Kaplan H. S., Musshoff K., Smithers D. W., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- Churchill W. H., Rocklin R. R., Moloney W. C., David J. R. In vitro evidence of normal lymphocyte function in some patients with Hodgkin's disease and negative delayed cutaneous hypersensitivity. Natl Cancer Inst Monogr. 1973 May;36:99–106. [PubMed] [Google Scholar]
- Eltringham J. R., Kaplan H. S. Immunodeficiency in Hodgkin disease. Birth Defects Orig Artic Ser. 1975;11(1):278–288. [PubMed] [Google Scholar]
- Faguet G. B. Quantitation of immunocompetence in Hodgkin's disease. J Clin Invest. 1975 Oct;56(4):951–957. doi: 10.1172/JCI108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld R., Bodey G. P., Rodriguez V., Luna M. Causes of death in patients with malignant lymphoma. Am J Med Sci. 1974 Aug;268(2):97–106. doi: 10.1097/00000441-197408000-00003. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., DeHoratius R., Israel H., Peake G. T., Messner R. P. Suppressor cell function in sarcoidosis. Ann Intern Med. 1979 Feb;90(2):169–173. doi: 10.7326/0003-4819-90-2-169. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P. Sensitivity of lymphocytes to prostaglandin E2 increases in subjects over age 70. J Clin Invest. 1979 Aug;64(2):434–439. doi: 10.1172/JCI109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFBRAND B. I. HODGKIN'S DISEASE AND HYPOGAMMAGLOBULINAEMIA: A RARE ASSOCIATION. Br Med J. 1964 May 2;1(5391):1156–1158. doi: 10.1136/bmj.1.5391.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C. Leukemia in patients treated for Hodgkin's disease. N Engl J Med. 1978 Apr 13;298(15):853–854. doi: 10.1056/NEJM197804132981516. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Oppenheim J. J. Impaired in vitro lymphocyte transformation in Hodgkin's disease. N Engl J Med. 1965 Nov 4;273(19):1006–1012. doi: 10.1056/NEJM196511042731903. [DOI] [PubMed] [Google Scholar]
- Hillinger S. M., Herzig G. P. Impaired cell-mediated immunity in Hodgkin's disease mediated by suppressor lymphocytes and monocytes. J Clin Invest. 1978 Jun;61(6):1620–1627. doi: 10.1172/JCI109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P., Johansson B. Impaired phytohaemagglutinin-induced cytotoxicity in vitro of lymphocytes from patients with Hodgkin's disease or chronic lymphatic leukaemia. Clin Exp Immunol. 1967 May;2(3):351–360. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Tosato G., Blaese R. M., Broder S., Magrath I. T. Polyclonal immunoglobulin secretion by human B lymphocytes exposed to Epstein-Barr virus in vitro. J Immunol. 1979 Apr;122(4):1310–1313. [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Laughter A. H., Lidsky M. D., Twomey J. J. Suppression of immunoglobulin synthesis by monocytes in health and in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1979 Dec;14(4):435–440. doi: 10.1016/0090-1229(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Laughter A. H., Twomey J. J. Suppression of lymphoproliferation by high concentrations of normal human mononuclear leukocytes. J Immunol. 1977 Jul;119(1):173–179. [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- MILLER D. G., LIZARDO J. G., SNYDERMAN R. K. Homologous and heterologous skin transplantation in patients with lymphomatous disease. J Natl Cancer Inst. 1961 Mar;26:569–583. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. F., Jr, Sugerman H., Shea W., Fleegler E. An extracorporeal device to treat barbiturate poisoning. Use of anion-exchange resins in dogs. JAMA. 1966 Jul 11;197(2):118–120. [PubMed] [Google Scholar]
- Reinherz E. L., Rubinstein A., Geha R. S., Strelkauskas A. J., Rosen F. S., Schlossman S. F. Abnormalities of immunoregulatory T cells in disorders of immune function. N Engl J Med. 1979 Nov 8;301(19):1018–1022. doi: 10.1056/NEJM197911083011902. [DOI] [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Schechter G. P., Soehnlen F. Monocyte-mediated inhibition of lymphocyte blastogenesis in Hodgkin disease. Blood. 1978 Aug;52(2):261–271. [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Bankhurst A. D., Williams R. C., Jr Studies of cell subpopulations mediating mitogen hyporesponsiveness in patients with Hodgkin's disease. J Clin Invest. 1978 Jan;61(1):55–63. doi: 10.1172/JCI108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R., Weitzman S. A., Aisenberg A. C., Weinstein H. J., Schiffman G. Impaired antibody response to pneumococcal vaccine after treatment for Hodgkin's disease. N Engl J Med. 1978 Aug 31;299(9):442–448. doi: 10.1056/NEJM197808312990903. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Lazar S., Douglass C. C. Reactivity of lymphocytes from primary neoplasms of lymphoid tissues. Cancer. 1976 Aug;38(2):740–747. doi: 10.1002/1097-0142(197608)38:2<740::aid-cncr2820380217>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Sharkey O., Jr, Brown J. A., Laughter A. H., Jordan P. H., Jr Cellular requirements for the mitotic response in allogeneic mixed leukocyte cultures. J Immunol. 1970 Apr;104(4):845–853. [PubMed] [Google Scholar]
- Wagener D. J., Van Munster P. J., Haanen C. The immunoglobulins in Hodgkin's disease. Eur J Cancer. 1976 Sep;12(9):683–688. doi: 10.1016/0014-2964(76)90016-5. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Aisenberg A. C., Siber G. R., Smith D. H. Impaired humoral immunity in treated Hodgkin's disease. N Engl J Med. 1977 Aug 4;297(5):245–248. doi: 10.1056/NEJM197708042970504. [DOI] [PubMed] [Google Scholar]
- Young R. C., Corder M. P., Haynes H. A., DeVita V. T. Delayed hypersensitivity in Hodgkin's disease. A study of 103 untreated patients. Am J Med. 1972 Jan;52(1):63–72. doi: 10.1016/0002-9343(72)90008-3. [DOI] [PubMed] [Google Scholar]


