Abstract
Objective:
To address the incidence of varicella-zoster virus (VZV) infection in patients with biopsy-negative giant cell arteritis (GCA), we examined archived biopsy-negative temporal arteries from subjects with clinically suspected GCA for the presence of VZV antigen.
Methods:
Formalin-fixed, paraffin-embedded temporal arteries that were pathologically negative for GCA and normal temporal arteries were analyzed immunohistochemically for VZV and herpes simplex virus-1 (HSV-1) antigen.
Results:
Five (21%) of 24 temporal arteries from patients who were clinically suspect but biopsy negative for GCA revealed VZV but not HSV-1 by immunohistochemical analysis. Thirteen normal temporal arteries did not contain VZV or HSV-1 antigen. All 5 subjects whose temporal arteries contained VZV antigen presented with clinical and laboratory features of GCA and early visual disturbances.
Conclusion:
Multifocal VZV vasculopathy can present with the full spectrum of clinical features and laboratory abnormalities characteristically seen in GCA.
Giant cell arteritis (GCA) is a disease of the elderly characterized by headache/head pain, scalp tenderness, jaw or tongue claudication, polymyalgia rheumatica, fever, night sweats, fatigue, and weight loss. Laboratory abnormalities include elevated erythrocyte sedimentation rates (ESRs) or C-reactive protein (CRP). Although not usually a presenting symptom, visual disturbances occur frequently. Because vision loss is often severe, GCA is a medical emergency. Temporal artery (TA) biopsy, the gold standard for diagnosis, reveals necrotizing vasculitis predominantly in the arterial media, although all layers may be involved. Notably, many patients with suspected GCA have normal TA biopsy,1 suggesting that other disorders may present similarly.
We showed that multifocal varicella-zoster virus (VZV) vasculopathy caused sudden vision loss in clinically suspect cases of GCA.2–4 Importantly, all patients had ischemic optic neuropathy and elevated ESRs and/or CRPs. Remarkably, 2 patients had no zoster history. Corticosteroid treatment failed to improve or worsened vision whereas treatment with antiviral agents often improved or stabilized vision. Because VZV is not present in TAs of patients with pathologically verified GCA,5,6 these cases identified a novel nosologic entity of multifocal VZV vasculopathy with TA infection and involvement of the ophthalmic, ciliary, or retinal arteries. Despite the confounding overlapping clinical features and laboratory abnormalities among these patients and those with GCA, the etiology may be confirmed by careful pathologic and virologic analysis of the ipsilateral TA.
To address the incidence of VZV infection in patients with biopsy-negative GCA, we searched for VZV antigen in archived TAs from subjects with clinically suspected GCA whose biopsies were pathologically negative.
METHODS
TAs examined.
Twenty-four archived formalin-fixed, paraffin-embedded (FFPE) TA specimens from subjects biopsied for possible GCA and found pathologically negative were obtained from the Department of Pathology at the University of Colorado Hospital submitted for pathologic diagnosis by neurologists, neuro-ophthalmologists, ophthalmologists, and rheumatologists from 2007 to 2012. The portion of the superficial TA that is biopsied is determined clinically by palpation to identify tenderness or nodularity. In addition, 13 normal TAs from 7 normal subjects (4 men and 3 women, aged 40–62 years, 3 of whom died of sepsis and the others of bowel ischemia, acute myelocytic leukemia, burn, and coronary artery disease) were obtained postmortem from the Department of Pathology at the University of Colorado Hospital and were FFPE.
Standard protocol approvals, registrations, and patient consents.
Analysis of archived TA specimens is considered to be a nonhuman exempt protocol because specimens are archived, deidentified, and coded. Similarly, normal TAs obtained at autopsy were deidentified and deemed exempt from review by the Colorado Multiple Institutional Review Board.
Immunohistochemistry.
From each FFPE TA, 100 (5-μm) sections were cut and baked on nonadhesive positively charged glass slides for 15 minutes at 65°C. Every fifth section was analyzed by immunohistochemistry for the presence of VZV gene 63 protein. Sections were deparaffinized in 100% xylene (3 times for 5 minutes each time) followed by 100% ethanol (3 times for 5 minutes each time). After sequential dipping in 95%, 70%, and 50% ethanol, sections were placed in distilled water, blocked in phosphate-buffered saline (PBS) containing 5% normal rabbit serum for 1 hour, washed 3 times with PBS, and incubated with a 1:5,000 dilution of primary rabbit polyclonal anti-VZV gene 63 antibody7 for 2 hours at room temperature or with a 1:1,000 dilution of rabbit anti–herpes simplex virus-1 (HSV-1) antibody (Dako, Carpinteria, CA), or with normal rabbit serum. Anti-VZV antibody does not stain uninfected human brain, arteries, lymph nodes, tonsils, liver, human brain vascular adventitial fibroblasts, smooth muscle cells or endothelial cells, human fetal lung fibroblasts, melanoma cells, or retinal pigment epithelial cells. Importantly, absorption of antibody with type A, B, AB, or O human red blood cells did not reduce staining of VZV-infected cells. Positive controls for the 2 antiviral antibodies were provided by cadaveric cerebral arteries infected ex vivo with VZV or HSV-1, respectively, and prepared as FFPE specimens 14 days postinfection. After incubation with primary antibody, sections were rinsed 3 times with PBS, followed by incubation with a 1:1,000 dilution of secondary biotinylated goat anti-rabbit antibody (Dako) for 1 hour, rinsed 3 times in PBS, and incubated with prediluted alkaline phosphatase–conjugated streptavidin (Becton-Dickinson, Franklin Lakes, NJ) for 1 hour. The color reaction was developed for 2 minutes using the fresh fuchsin substrate system (Dako) in the presence of levamisole (Dako) at a final concentration of 24 μg/mL. The presence of VZV or HSV-1 antigen (pink staining) was determined using light microscopy. In addition, when a VZV-positive TA section was identified, the remaining 95 sections spanning 500 μm from the same artery were further stained to determine how many sections contained VZV antigen.
RESULTS
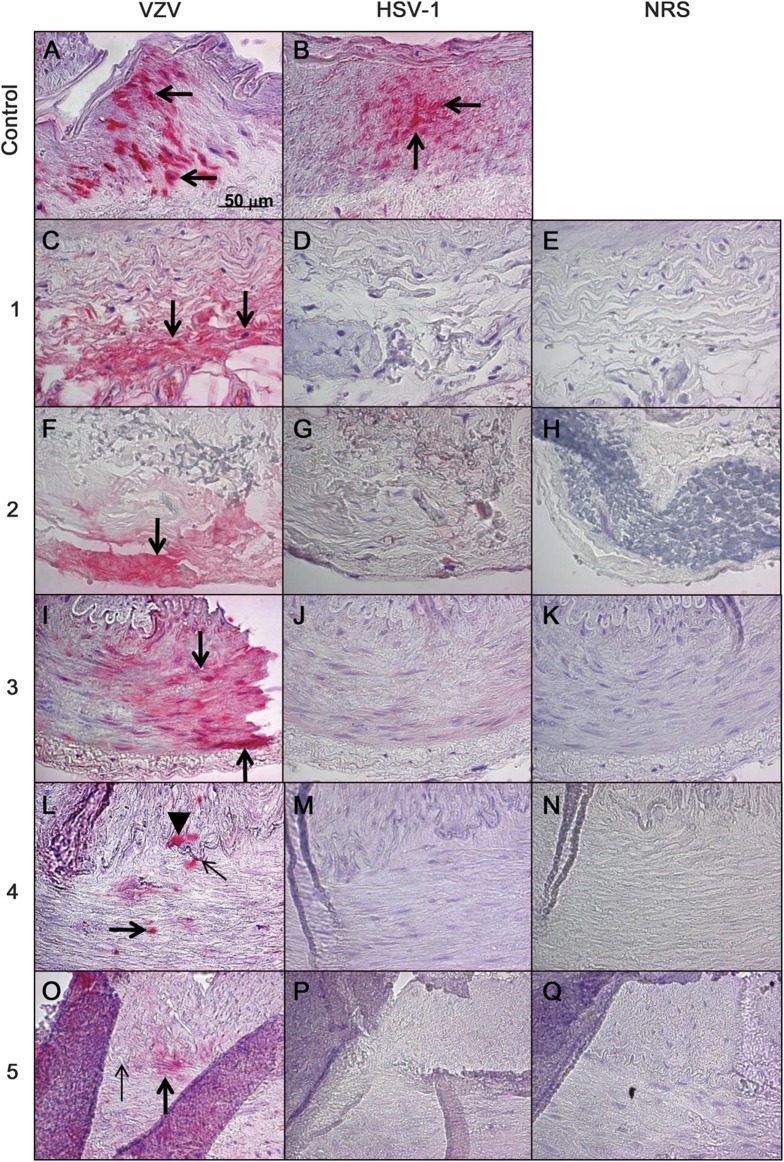
VZV antigen, but not HSV-1 antigen, was detected in 5 (21%) of 24 pathologically negative TAs from patients with clinically suspect GCA (figure 1). VZV was found exclusively in the arterial adventitia in 2 arteries, in the arterial media in another 2 arteries, and in both the media and intima in 1 artery. Further analysis of 95 additional sections from all 5 VZV-positive TAs revealed VZV antigen in 4, 4, 6, 4, and 2 different areas, respectively; figure 2 shows 4 positive areas in one of these arteries. None of the 5 subjects had a history of zoster. Similar analysis of 13 normal TAs obtained from 7 autopsy subjects revealed no VZV or HSV-1 antigen.
Figure 1. VZV antigen in biopsy-negative temporal arteries from subjects with clinically suspected GCA.
Positive control VZV-infected cadaveric human cerebral artery (A, arrows) shows VZV antigen (pink color). Five (21%) of 24 subjects with suspected GCA, but whose temporal arteries were pathologically GCA negative, contained VZV antigen. Subjects 1 and 2 had VZV antigen exclusively in the adventitia (C and F, respectively, arrows). Subject 3 had VZV antigen exclusively in the media (I, arrows). Subject 4 had VZV antigen in the media (L, thick arrow) and in the intima (L, arrowhead) adjacent to the internal elastic lamina (L, thin arrow). Subject 5 had VZV antigen exclusively in the media (O, thick arrow) adjacent to the internal elastic lamina (O, thin arrow). HSV-1 antigen was present in the positive control HSV-1–infected cadaveric cerebral artery (B, arrows), but not in any human temporal arteries, including those that contained VZV antigen (D, G, J, M, and P). No staining was seen when NRS was used as primary antibody on temporal arteries (E, H, K, N, and Q). None of 13 temporal arteries from 7 normal subjects contained VZV antigen (data not shown). Original magnification ×600. GCA = giant cell arteritis; HSV-1 = herpes simplex virus-1; NRS = normal rabbit serum; VZV = varicella-zoster virus.
Figure 2. Multiple areas of VZV antigen in a biopsy-negative temporal artery of a subject with clinically suspected giant cell arteritis.
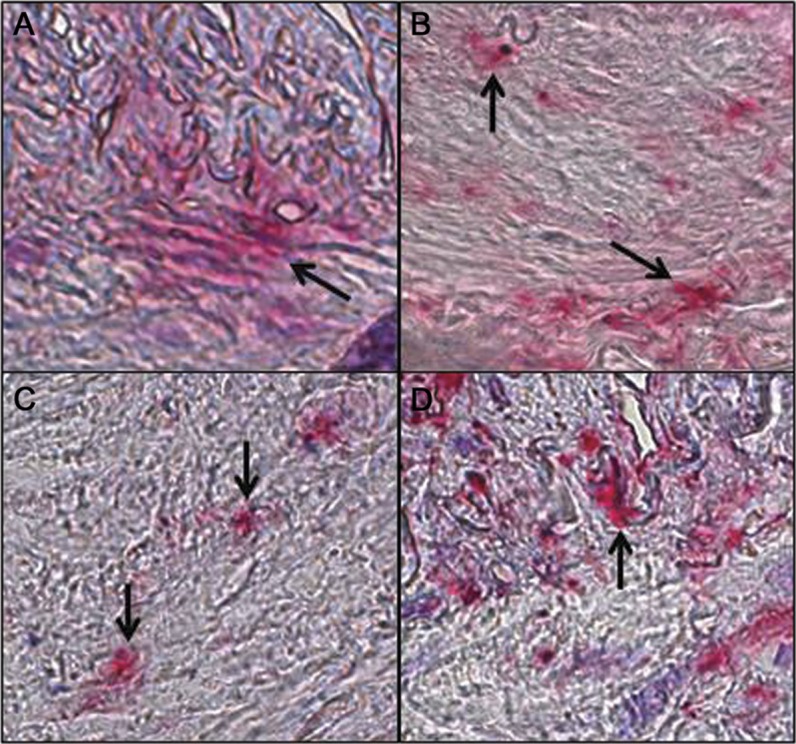
In a temporal artery that was found to contain VZV antigen, 95 consecutive 5-μm sections from that artery were further stained to determine how many sections contained VZV antigen. Arrows show 4 positive areas along a 500-μm length of temporal artery at position 20, 160, 250, and 480 μm (A–D, respectively). Original magnification ×600. VZV = varicella-zoster virus.
Of the 5 subjects whose TAs contained VZV, 4 were men 69 to 79 years of age, and the fifth was a 58-year-old woman (table). All 5 subjects (100%) had early visual disturbances. Three subjects had acute unilateral vision loss with histories and ophthalmologic examinations diagnostic of posterior ischemic optic neuropathy, anterior ischemic optic neuropathy (AION), or central retinal artery occlusion. Two subjects had transient visual disturbances, lasting up to 1 year in one subject, suggestive of episodic amaurosis fugax.
Table.
Clinical and laboratory features of subjects with multifocal VZV vasculopathy and temporal artery infection that mimic GCA
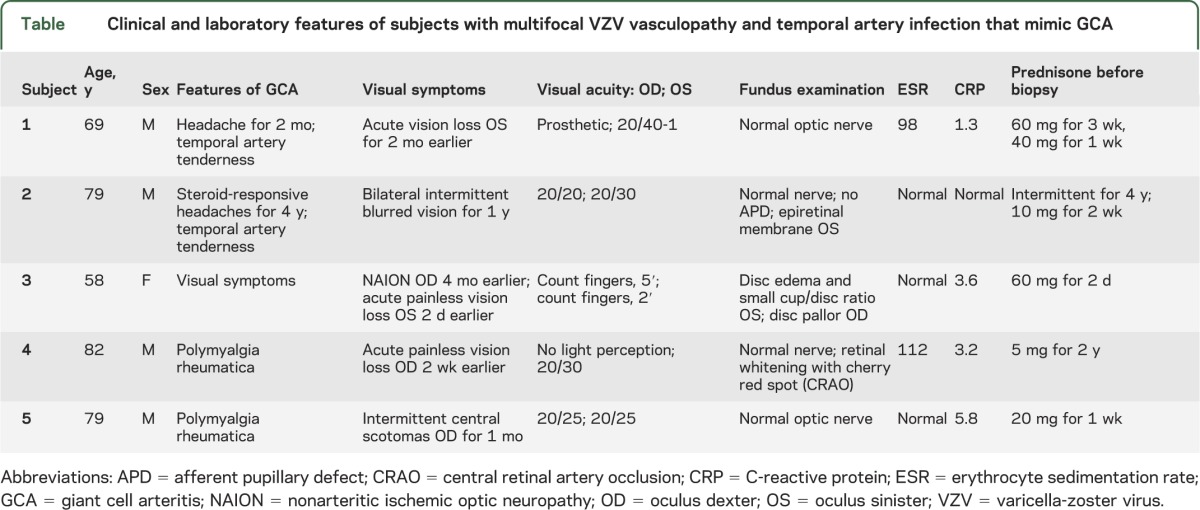
Three subjects (60%) had bilateral headache, 2 (40%) had TA tenderness, and 2 (40%) had a history of polymyalgia rheumatica. None had jaw claudication, fever, night sweats, or weight loss. All 5 subjects were taking corticosteroids at the time of TA biopsy (range: 2 days to 4 years before biopsy). Systemic inflammatory markers were characteristically elevated: 2 subjects (40%) had an elevated ESR and 4 (80%) had an elevated CRP.
The 19 subjects whose TAs did not contain VZV antigen comprised 9 men and 10 women 42 to 82 years of age. Of these 19 subjects, 14 (74%) had headaches, 2 (10%) had TA tenderness, 4 (21%) had unilateral visual disturbances, 4 (21%) had jaw claudication, 3 (16%) had polymyalgia rheumatica, 3 (16%) had fever, 6 (32%) had fatigue, 2 (10%) had night sweats, and 4 (21%) had weight loss. Eight (42%) were taking corticosteroids at the time of TA biopsy. Ten (53%) had elevated ESRs and 9 (47%) had elevated CRPs.
DISCUSSION
Based on our previous cases that demonstrated that GCA and multifocal VZV vasculopathy with TA infection have overlapping clinical features and laboratory abnormalities,2–4 we examined 24 TAs that were GCA negative pathologically for the presence of VZV and HSV antigen. Immunohistochemical examination revealed VZV antigen, but not HSV-1 antigen, in 5 (21%) of 24 TAs, indicating that VZV produced a multifocal vasculopathy affecting the ophthalmic and/or retinal arteries to cause visual disturbance, in addition to ipsilateral TA infection. Ischemic disease is likely explained by a thickened intima,8 resulting from inflammation predominantly in the adventitia.9 The absence of significant inflammation in VZV-infected TAs most likely reflects early treatment with corticosteroids.
A number of important features have emerged from analysis of these remarkable cases of multifocal VZV vasculopathy. First, VZV vasculopathy can present with the full spectrum of anterior visual pathway injuries observed in GCA. In our small sample of cases, we observed posterior ischemic optic neuropathy (table, subject 1), transient ischemia (table, subjects 2 and 5), AION (table, subject 3), and central retinal artery occlusion (table, subject 4). Although the central retinal artery does not possess an internal elastic lamina, 5% to 10% of central retinal artery occlusions are associated with thrombus formation from GCA.10 Of note, the prolonged intermittent transient loss of vision experienced by subject 2 indicates that multifocal VZV may have a protracted course. An even more protracted course was recently reported in a patient with multifocal VZV vasculopathy who experienced 5 strokes over a 2-year period after an initial presentation of ischemic optic neuropathy.11 Importantly, none of the subjects in this study had a history of zoster, thus adding another serious neuro-ophthalmologic disorder to the multiple conditions (chronic radiculopathy, meningoencephalitis, myelopathy, vasculopathy, cerebellitis, and retinitis) produced by VZV in the absence of rash.
Second, multifocal VZV vasculopathy may exhibit many of the same clinical features (headache, polymyalgia rheumatica, TA tenderness) and laboratory abnormalities (elevated ESR and CRP) seen in GCA. The overlap of clinical and laboratory abnormalities in the 2 disorders underscores the need for rapid, accurate diagnosis because patients with multifocal VZV vasculopathy require antiviral treatment.
Third, the association of multifocal VZV vasculopathy with AION indicates that virus reactivation may be associated with some cases of nonarteritic AION, a common cause of optic neuropathy over the age of 50 years (table, case 3). Evaluation of TAs from a prospective collection of nonarteritic AION patients for VZV antigen may identify a subgroup of individuals who would benefit from early antiviral treatment to improve visual outcomes and prevent recurrence in the fellow eye.
The relationship of steroid treatment to the 5 subjects with multifocal VZV vasculopathy whose TAs contained VZV antigen warrants discussion. We recognize that 3 of the 5 positive subjects had been taking steroids intermittently for months to years; thus, the possibility exists that VZV antigen in the TA resulted from steroid treatment. However, the other 2 positive subjects had been taking steroids for only 7 and 2 days, respectively. In contrast, among the subjects whose TAs did not contain VZV antigen, 5 of 8 individuals had been taking corticosteroids for 1 week to more than 3 months before biopsy. More importantly, all 5 subjects had visual and other symptoms, along with elevated ESRs and CRPs. The clinical features and laboratory abnormalities must be taken in context with the presence of VZV antigen in the TA. Thus, it is likely that these patients all had multifocal VZV vasculopathy that affected the retinal or ophthalmic artery as well as the TA. At the time of TA biopsy, multifocal VZV vasculopathy was not a diagnostic consideration, thus antiviral agents were not given.
Overall, our current findings indicate that in patients presenting with clinical symptoms (particularly early visual disturbances), signs, and laboratory abnormalities (particularly elevated CRP) consistent with GCA, the diagnosis of multifocal VZV vasculopathy is an important consideration and warrants examination of the TA not only for histopathologic features of GCA but also for immunohistochemical evidence of VZV antigen. In addition, detection of VZV DNA and anti-VZV immunoglobulin G antibody in CSF may also help to confirm the diagnosis of multifocal VZV vasculopathy in these patients as it did in a recent patient with VZV ischemic optic neuropathy and subclinical TA involvement.3 Finally, larger studies are needed to determine the spectrum of symptoms, signs, and laboratory abnormalities, as well as the characteristic histopathologic features of multifocal VZV vasculopathy with TA infection.
GLOSSARY
- AION
anterior ischemic optic neuropathy
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FFPE
formalin-fixed, paraffin-embedded
- GCA
giant cell arteritis
- HSV-1
herpes simplex virus-1
- PBS
phosphate-buffered saline
- TA
temporal artery
- VZV
varicella zoster virus
AUTHOR CONTRIBUTIONS
M.A. Nagel: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patents, acquisition of data, statistical analysis, study supervision or coordination, obtaining funding. J.L. Bennett: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, statistical analysis. N. Khmeleva, A. Choe, and A. Rempel: acquisition of data. P.J. Boyer: drafting/revising the manuscript for content, including medical writing for content, contribution of vital reagents/tools/patents. D. Gilden: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patents, acquisition of data, statistical analysis, study supervision or coordination, obtaining funding.
STUDY FUNDING
This work was supported in part by NIH grants AG006127 and AG032958 to D.G. and NS067070 to M.A.N. Ms. Rempel's work was supported by NIH grant R25 GM-083333.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Stuart RA. Temporary artery biopsy in suspected temporal arteritis: a five year survey. N Z Med J 1989;102:431–433 [PubMed] [Google Scholar]
- 2.Salazar R, Russman AN, Nagel MA, et al. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011;68:517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel MA, Russman AN, Feit DO, et al. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology 2013;80:220–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci 2013;325:180–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordborg C, Nordborg E, Petursdottir V, et al. Search for varicella zoster virus in giant cell arteritis. Ann Neurol 1998;44:413–414 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy PG, Grinfeld E, Esiri MM. Absence of detection of varicella-zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J Neurol Sci 2003;215:27–29 [DOI] [PubMed] [Google Scholar]
- 7.Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol 1995;69:3240–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology 2011;77:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel MA, Traktinskiy I, Kleinschmidt-DeMasters B, et al. VZV vasculopathy: immune characteristics virus-infected arteries. Neurology 2013;80:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller N, Newman N. Walsh & Hoyt’s Clinical Neuro-Ophthalmology, 5th ed Baltimore: Lippincott Williams & Wilkins; 1998:3758–3759 [Google Scholar]
- 11.Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2 years' duration. J Neurol Sci 2012;323:245–247 [DOI] [PMC free article] [PubMed] [Google Scholar]