Abstract
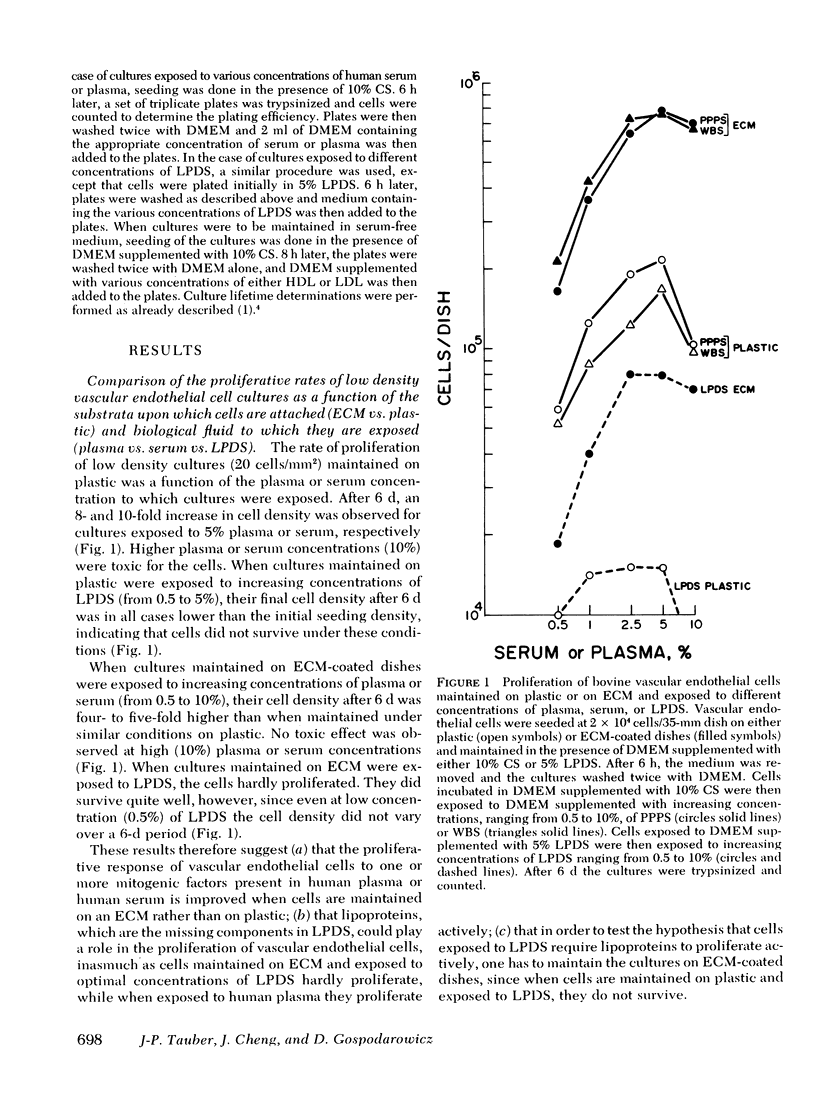
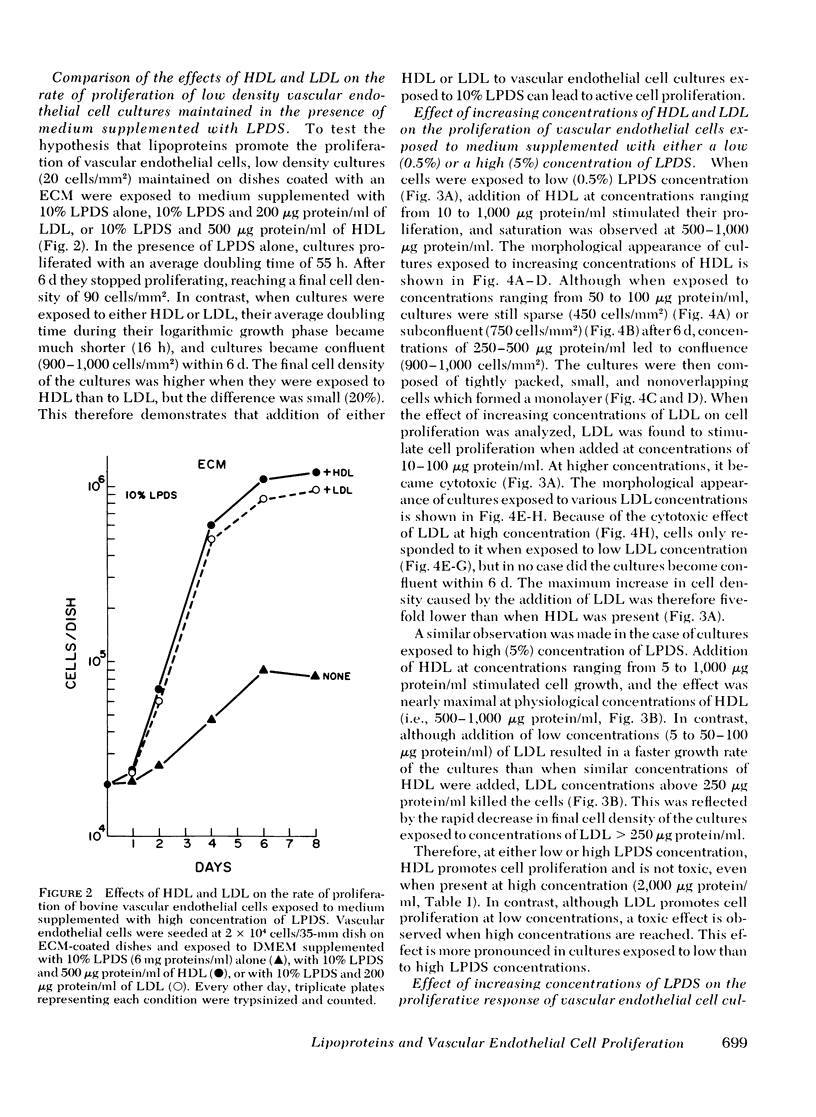
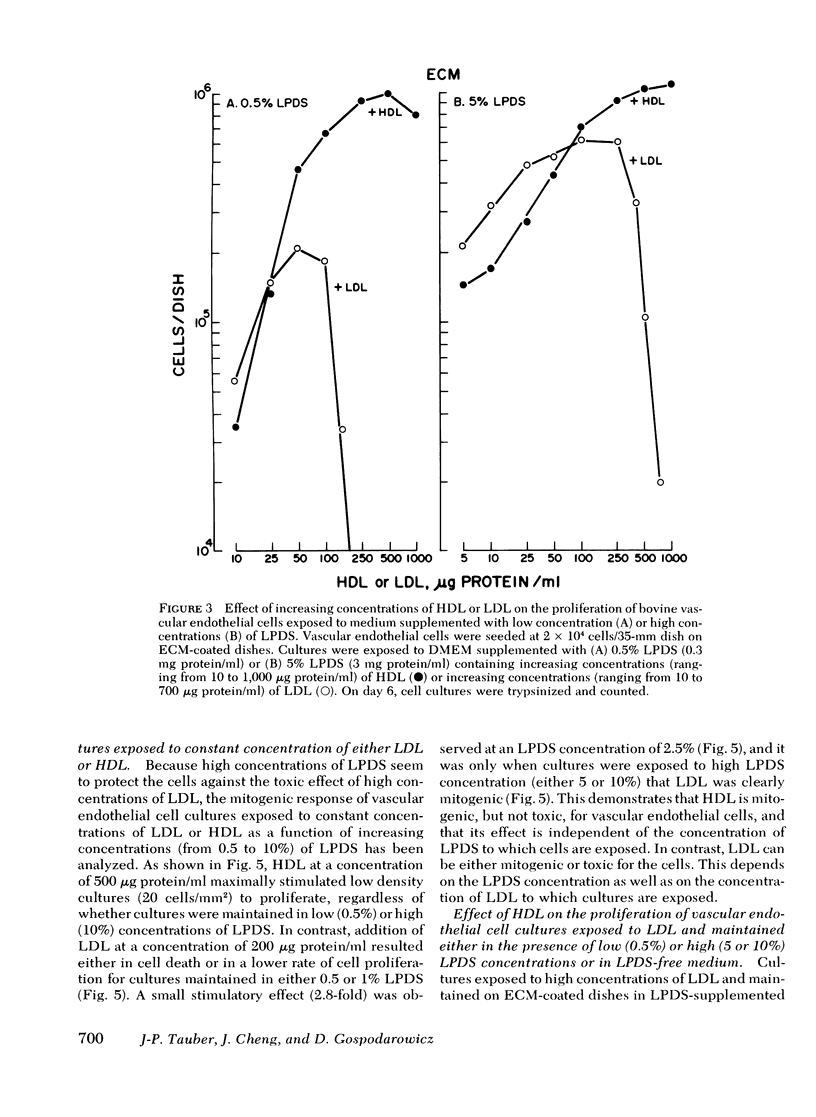
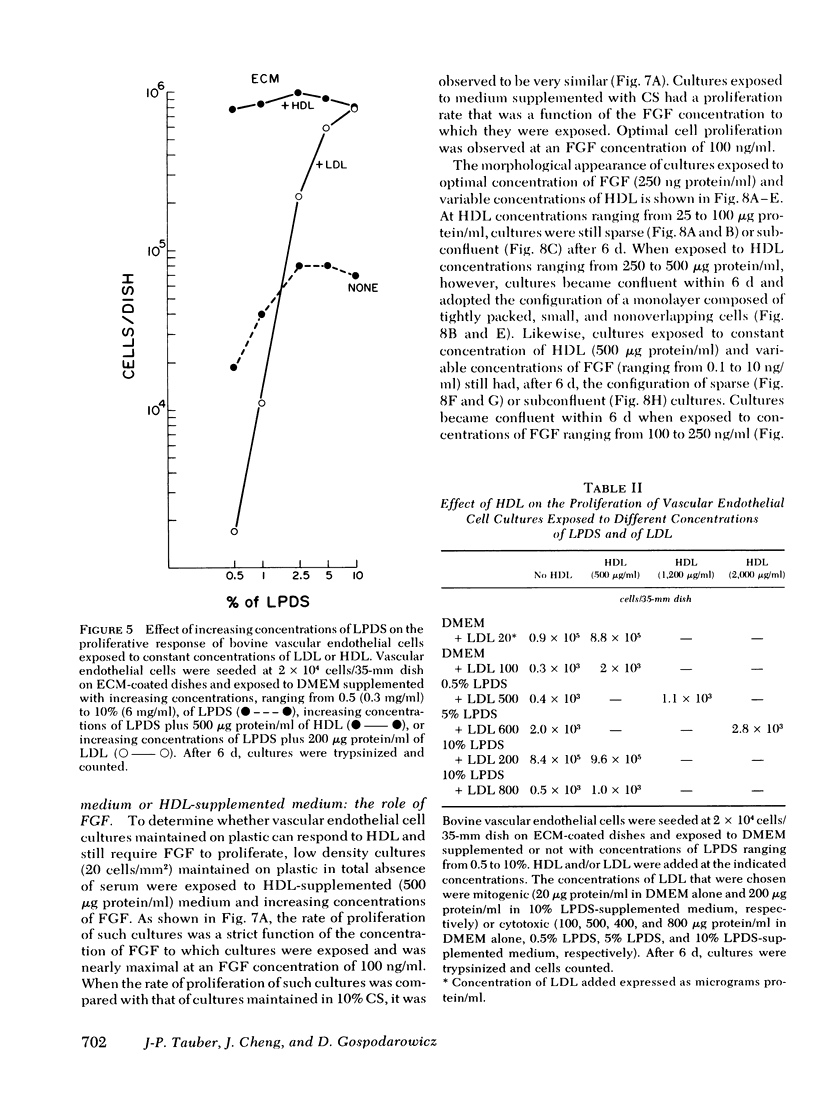
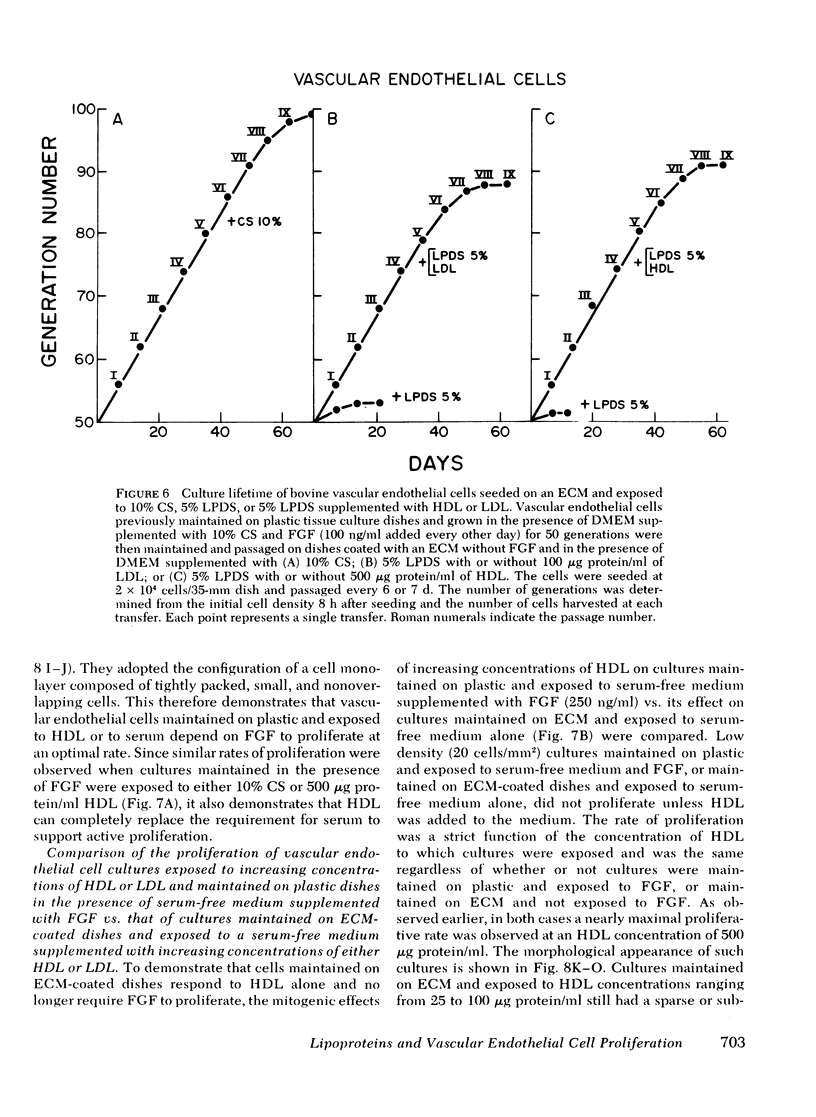
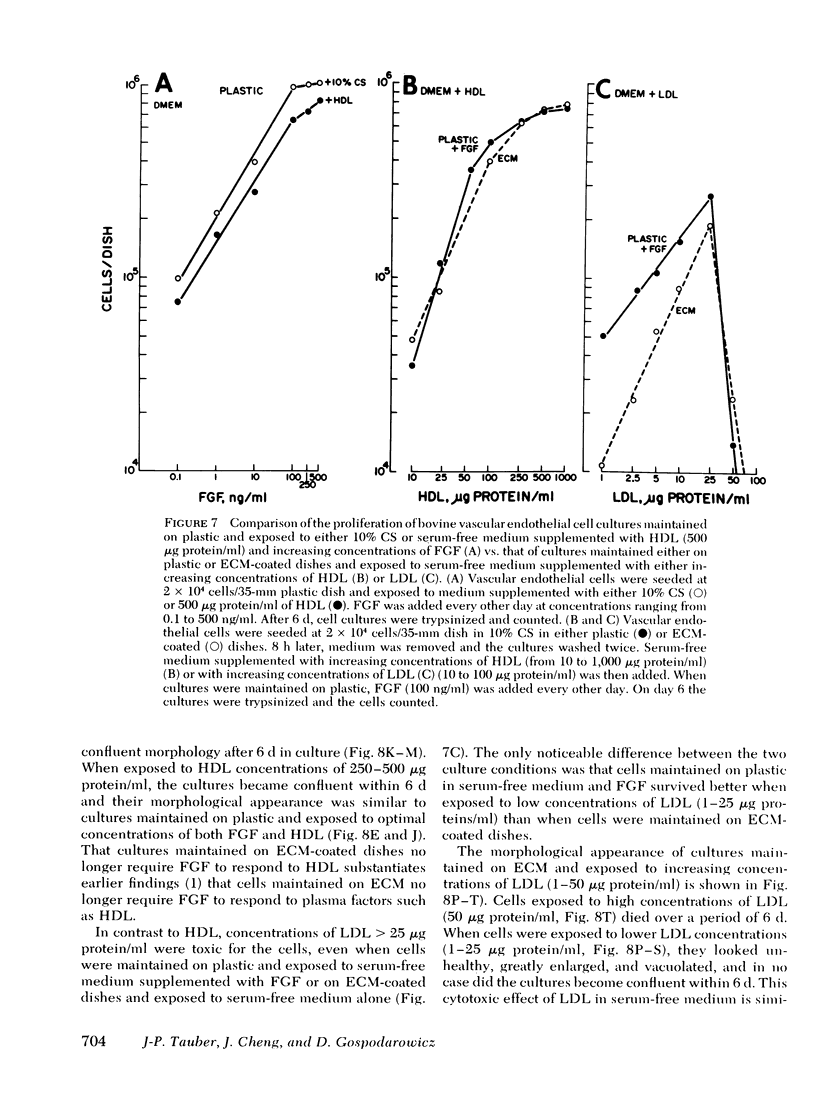
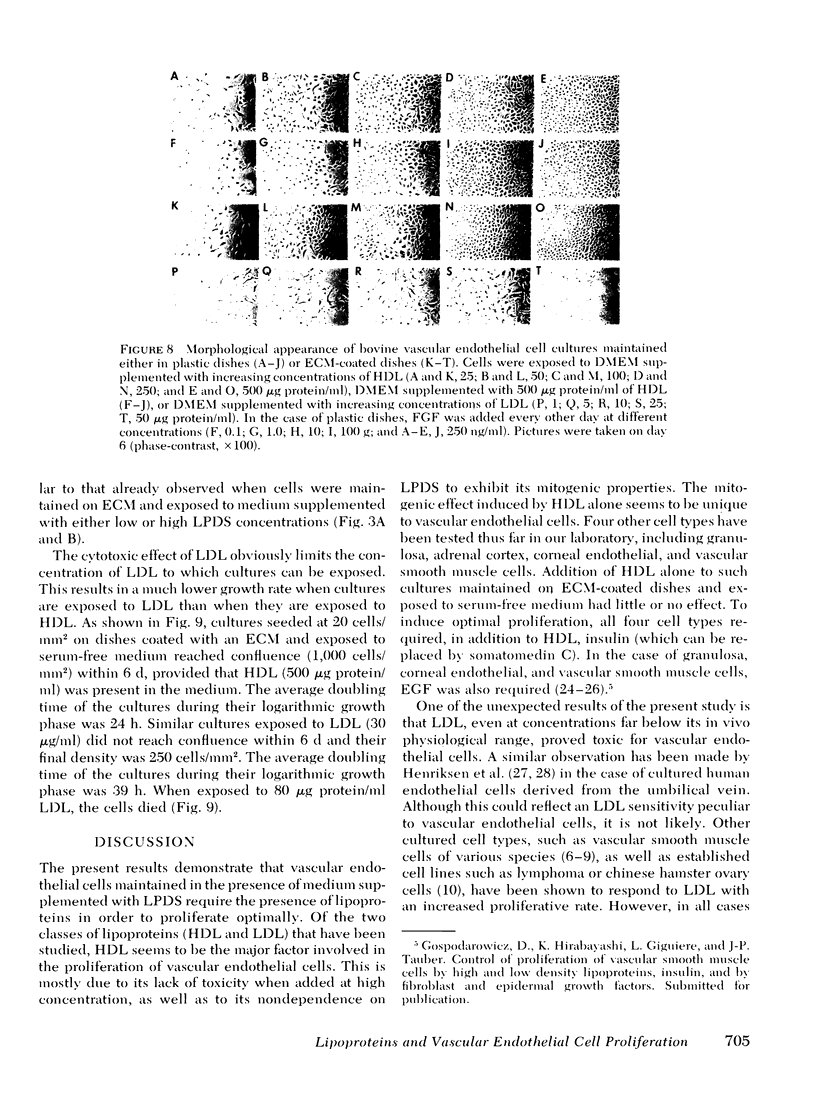
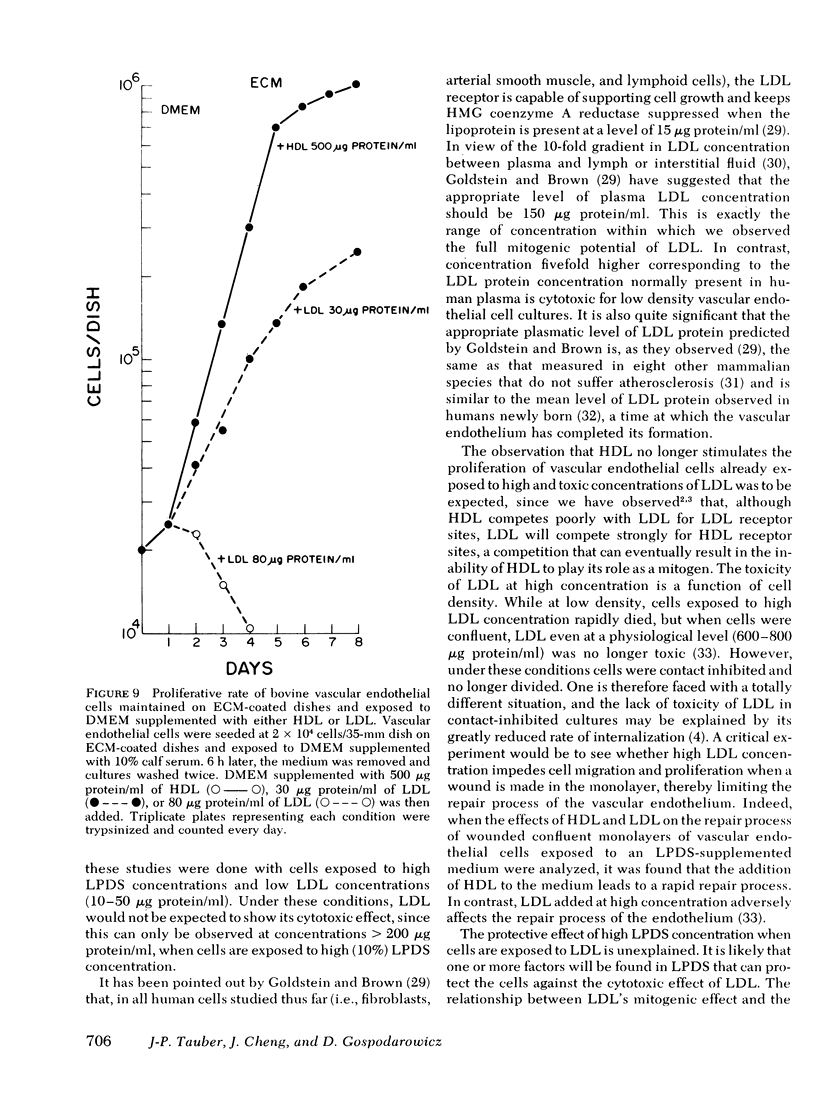
Bovine vascular endothelial cells maintained on dishes coated with an extracellular matrix and exposedto medium supplemented with lipoprotein-deficient serum (LPDS) require the presence of lipoprotein to proliferate optimally. High density lipoprotein (HDL) seems to be the major factor involved in the proliferation of vascular endothelial cells. This is mostly due to its lack of toxicity when added at high concentration, as well as to its nondependence on LPDS to exhibit its mitogenic properties. Therefore, HDL at physiological concentrations (1,000--1,500 microgram protein/ml) can fully replace serum. Low density lipoprotein, unlike HDL, has a biphasic effect. Although mitogenic for vascular endothelial cells when added at low concentration, once physiological concentrations are reached it becomes toxic for the cells. Moreover, and in contrast with HDL, the mitogenic effect of low density lipoprotein was found to be a function of the LPDS concentration to which cultures were exposed. The substrate upon which cultures are maintained has been found to be an important factor if a mitogenic effect of HDL is to be observed. When maintained on plastic, cells proliferate poorly in response to HDL unless fibroblast growth factor is added to the medium. In contrast, when maintained on extracellular matrix, an optimal growth rate is induced by HDL, even in the absence of fibroblast growth factor. This suggests that, in vivo, the integrity of the basement membrane upon which endothelial cells rest and migrate is an important factor in determining the cells response to lipoproteins present in plasma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. G., Mahley R., Assmann G. Swine aortic smooth muscle in tissue culture. Some effects of purified swine lipoproteins on cell growth and morphology. Circ Res. 1976 Sep;39(3):415–424. doi: 10.1161/01.res.39.3.415. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Vlodavsky I., Gospodarowicz D., Fielding C. J. Effect of contact inhibition on the regulation of cholesterol metabolism in cultured vascular endothelial cells. J Biol Chem. 1979 Feb 10;254(3):749–755. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Helgeson J. A., Brown M. S. Inhibition of cholesterol synthesis with compactin renders growth of cultured cells dependent on the low density lipoprotein receptor. J Biol Chem. 1979 Jun 25;254(12):5403–5409. [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S., Braun D. L. Control of proliferation of bovine vascular endothelial cells. J Cell Physiol. 1977 Jun;91(3):377–385. doi: 10.1002/jcp.1040910307. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to cultured endothelial cells induced by low density lipoproteins: protection by high density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):369–375. doi: 10.3109/00365517909106121. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Layman D. L., Jelen B. J., Illingworth D. R. Inability of serum from abetalipoproteinemic subjects to stimulate proliferation of human smooth muscle cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1511–1515. doi: 10.1073/pnas.77.3.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mills G. L., Taylaur C. E. The distribution and composition of serum lipoproteins in eighteen animals. Comp Biochem Physiol B. 1971 Oct;40(2):489–501. doi: 10.1016/0305-0491(71)90234-3. [DOI] [PubMed] [Google Scholar]
- Reckless J. P., Weinstein D. B., Steinberg D. Lipoprotein and cholesterol metabolism in rabbit arterial endothelial cells in culture. Biochim Biophys Acta. 1978 Jun 23;529(3):475–487. doi: 10.1016/0005-2760(78)90091-7. [DOI] [PubMed] [Google Scholar]
- Reichl D., Simons L. A., Myant N. B., Pflug J. J., Mills G. L. The lipids and lipoproteins of human peripheral lymph, with observations on the transport of cholesterol from plasma and tissues into lymph. Clin Sci Mol Med. 1973 Sep;45(3):313–329. doi: 10.1042/cs0450313. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Tsnag R. C., Glueck C. J., Fallat R. W., Mellies M. Neonatal familial hypercholesterolemia. Am J Dis Child. 1975 Jan;129(1):83–91. [PubMed] [Google Scholar]




