Abstract
Objective:
We sought to determine the relationship of greater adherence to Mediterranean diet (MeD) and likelihood of incident cognitive impairment (ICI) and evaluate the interaction of race and vascular risk factors.
Methods:
A prospective, population-based, cohort of individuals enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study 2003–2007, excluding participants with history of stroke, impaired cognitive status at baseline, and missing data on Food Frequency Questionnaires (FFQ), was evaluated. Adherence to a MeD (scored as 0–9) was computed from FFQ. Cognitive status was evaluated at baseline and annually during a mean follow-up period of 4.0 ± 1.5 years using Six-item-Screener.
Results:
ICI was identified in 1,248 (7%) out of 17,478 individuals fulfilling the inclusion criteria. Higher adherence to MeD was associated with lower likelihood of ICI before (odds ratio [lsqb]OR[rsqb] 0.89; 95% confidence interval [lsqb]CI[rsqb] 0.79–1.00) and after adjustment for potential confounders (OR 0.87; 95% CI 0.76–1.00) including demographic characteristics, environmental factors, vascular risk factors, depressive symptoms, and self-reported health status. There was no interaction between race (p = 0.2928) and association of adherence to MeD with cognitive status. However, we identified a strong interaction of diabetes mellitus (p = 0.0134) on the relationship of adherence to MeD with ICI; high adherence to MeD was associated with a lower likelihood of ICI in nondiabetic participants (OR 0.81; 95% CI 0.70–0.94; p = 0.0066) but not in diabetic individuals (OR 1.27; 95% CI 0.95–1.71; p = 0.1063).
Conclusions:
Higher adherence to MeD was associated with a lower likelihood of ICI independent of potential confounders. This association was moderated by presence of diabetes mellitus.
Dementia is a common disorder among older adults, with a prevalence of approximately 15% for individuals aged >70 years.1 Currently there are no preventive or curative pharmaceutical measures.2 Epidemiologic studies suggest that dietary factors are related to a lower risk of Alzheimer disease (AD), but reports are inconsistent.3–10 The Mediterranean diet (MeD) is a dietary pattern characterized by high consumption of plant foods or olive oil and low intake of saturated fat, meat, or dairy products.11 It has recently received increased attention since high adherence to MeD has been associated with longer survival,12,13 reduced risk of cardiovascular12,13 or cancer14 mortality, and lower likelihood of AD.15−18
Given that black subjects were underrepresented in the cohorts that evaluated the association of adherence to MeD with incident cognitive impairment,15–18 there are limited data regarding this potential association in black subjects. Moreover, there is accruing evidence indicating higher prevalence of dementia among nonwhite persons,19,20 while it has been suggested that disproportionately high rates of dementia among black subjects may be attributed to a higher prevalence of hypertension, obesity, diabetes, and stroke.21,22 Notably, racial disparities in fruit and vegetable intake have been reported.23,24
In view of these considerations, we sought to determine the association between higher adherence to MeD and lower likelihood of incident cognitive impairment using the population-based cohort of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.25 We also evaluated the potential interaction of race and vascular risk factors on the association of adherence to MeD with incident impaired cognitive function.
METHODS
Study design.
REGARDS is a large, geographically dispersed, national, population-based, longitudinal cohort study with oversampling of black subjects and persons from the Stroke Belt region of the United States, an area that has stroke mortality rates higher than the rest of the United States.25 From January 2003 through October 2007, a total of 30,239 individuals 45 years or older, self-identified as non-Hispanic black or white, were enrolled. Methodologic details are available elsewhere.25–27
Standard protocol approvals, registration, and consents.
The study methods have been reviewed and approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all participants involved in the study.
Data collection and definitions.
Cognitive assessment.
The Six-item Screener (SIS) was used for assessment of cognitive function.28 Cognitive assessments were performed at baseline and during the follow-up period on an annual basis. The main outcome of the present analyses is incident cognitive impairment defined by change between participants’ first and last scores on the SIS. More specifically, incident cognitive impairment was defined as a shift from intact cognitive screening status at the first assessment (SIS score 5–6) to impaired cognitive screening status at the latest available assessment (SIS score ≤4).28 Detailed description of previous validation of SIS score is available in e-Methods on the Neurology® Web site at www.neurology.org.
Dietary assessment and MeD.
Average food consumption information at baseline was obtained using the self-administered Block 98 Food Frequency Questionnaire (FFQ),29 which was left with each participant during the in-person visit with instructions for completion and a stamped envelope in which to return the questionnaire. Two unannounced 24-hour recalls (weekday and weekend) were used in addition to a second FFQ administered within a year to validate the FFQ. Foods for the FFQ were selected based on commonly reported foods in the National Health and Nutrition Survey. The diet score was analyzed in a median (low adherence range 0–4; high adherence range 5–9) and tertile split (low adherence 0–3; moderate adherence 4–5; high adherence 6–9).16–18 Additional details regarding the construction of MeD score are available in e-Methods.
Covariates.
Covariates included in the present analysis were the following variables that have been previously associated with incident cognitive impairment15–17: age, race, sex, region of residence, body mass index (BMI), waist circumference, household income, education, smoking status, alcohol use, physical activity level, history of heart disease, diabetes mellitus (DM), atrial fibrillation, systolic blood pressure (SBP), diastolic blood pressure (DBP), high cholesterol, antihypertensive regimen (specific drug classes), perceived general health status, and depressive symptoms evaluated using Center for Epidemiological Studies–Depression–4-item.30 The definitions of these variables have been described.25–27
Participants.
This analysis included only REGARDS participants who during the baseline interview had reported having no prior history of stroke, did not have impaired cognitive status at baseline assessment (score ≤4 on SIS),28 had at least 2 cognitive screening assessments, did not have missing data with respect to demographic or food intake variables, and had a daily caloric intake ranging from 600 to 5,000 calories. We considered only those who answered at least 85% of the FFQ with caloric intakes between 600 and 5,000 to be eligible for this analysis. Of the 21,033 participants with FFQ data, we excluded those reporting prior stroke (n = 1,159), those for whom we did not have at least 2 cognitive assessments (n = 1,147), and those who were cognitively impaired (SIS score ≤4) at baseline (n = 1,249), resulting in a cohort of 17,478 participants. Follow-up was through September 2010. A flow diagram of the present study reporting numbers of individuals at each stage of the study and providing reasons for nonparticipation at each stage is shown in figure 1.
Figure 1. Flow diagram of the study population.
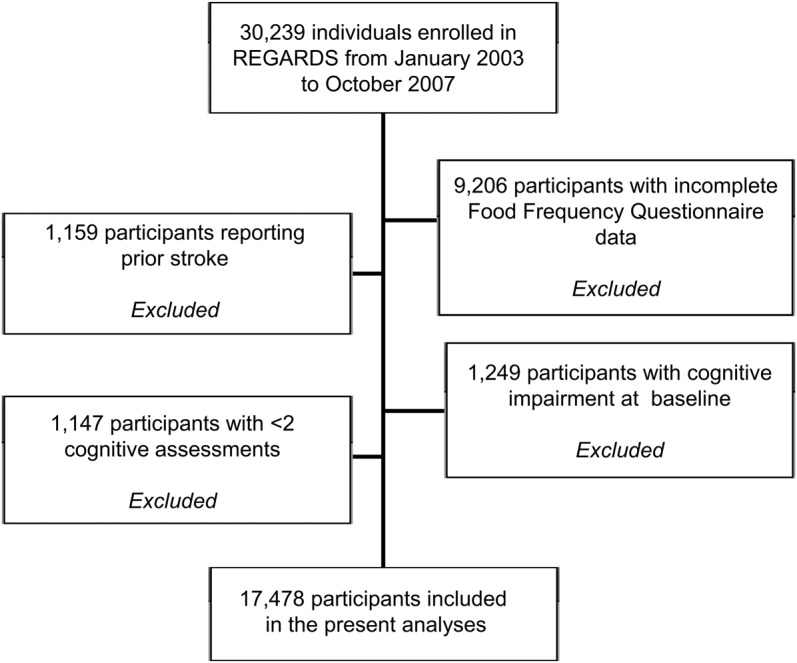
REGARDS = Reasons for Geographic and Racial Differences in Stroke.
Statistical analyses.
The 2-tailed Pearson χ2 test for categorical variables and Student t test or Mann-Whitney U test for continuous variables were used to assess intergroup differences between participants with high and low adherence to MeD score. Because the SIS was validated using dichotomous outcomes rather than continuous scores,28 logistic regression was selected to evaluate the relationship between adherence to MeD (dichotomized MeD score) and incident cognitive impairment using a set of incremental models as previously described.31 Moreover, we repeated our analyses in this specific REGARDS dataset using proportional hazards models (in order to take into account the effect of time) and obtained similar results to the findings of logistic regression analyses.31 We also performed a sensitivity analysis using a more stringent definition of incident impairment, in which an SIS score in the impaired range (score of 4 or fewer correct) was required at the 2 latest assessments. Additional information regarding the incremental logistic regression models is available on the Neurology® Web site at www.neurology.org. Analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
The sample of 17,478 individuals included in the present analyses had a mean age of 64.4 ± 9.1 years (range 45–98 years, normal distribution) and was 31% black (n = 5,368), 43% male (n = 7,548), and 56% (n = 9,832) from the Stroke Belt region. DM was prevalent in 2,913 participants (17%). Individuals who were excluded from the analyses differed (p < 0.05) from included subjects in terms of income, education, and race. Included individuals were more likely to have graduated college, have an income above $75,000, and self-identify as white. There were no other differences between individuals excluded and included in the analyses in terms of age, sex, location of residence, smoking and exercise habits, vascular risk factors, depressive symptoms, and self-reported health status.
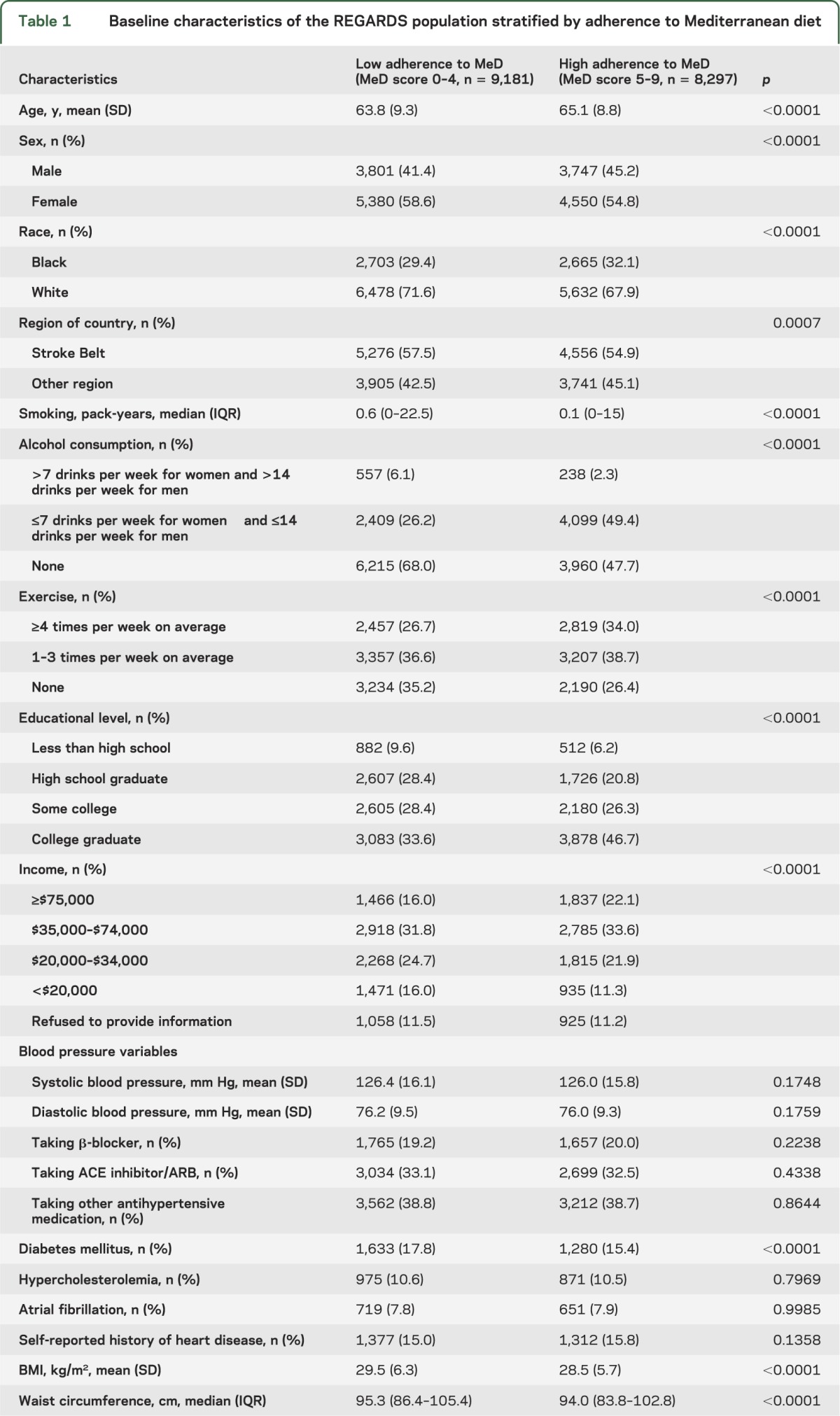
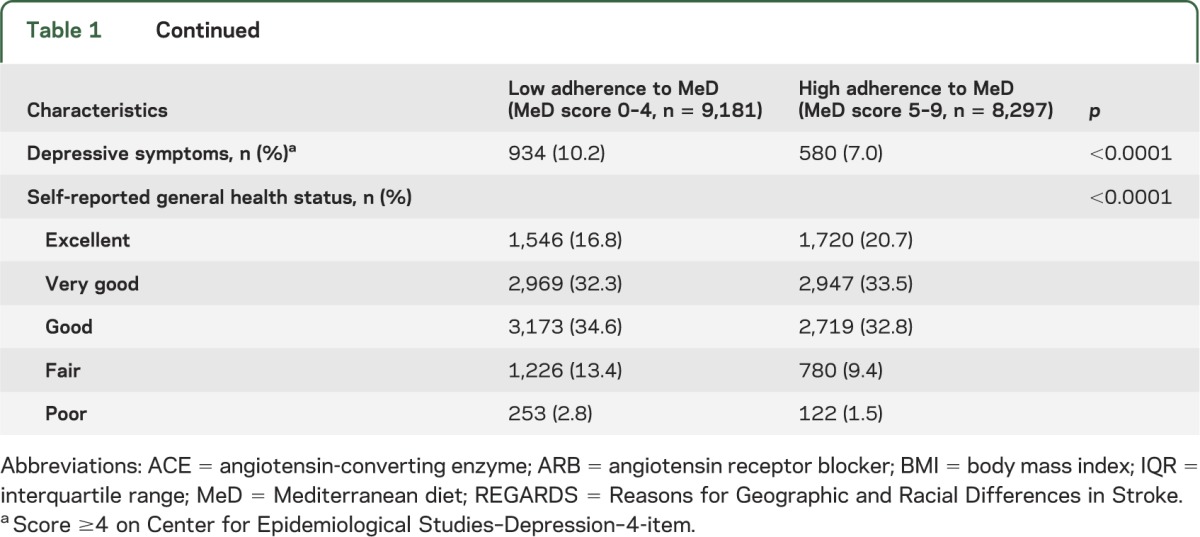
The MeD score ranged from 0 to 9 and had a normal distribution with 42% of participants (n = 7,348) having a score of 4–5. The mean MeD score was 4.4 ± 1.7. A total of 9,181 individuals (53%) had a low adherence to MeD (MeD score 0–4). Demographic characteristics, environmental and vascular risk factors, and prevalence of depressive symptoms in participants with low and high adherence to MeD are presented in table 1. Greater MeD (MeD score 5–9) adherence was associated with older age, male sex, black race, other region than the Stroke Belt, fewer pack-years of smoking, less prevalent moderate/heavy alcohol consumption, more regular exercise, higher income and educational level, less prevalent DM, lower BMI, smaller waist circumference, lower prevalence of depressive symptoms, and higher prevalence of excellent self-reported health status.
Table 1.
Baseline characteristics of the REGARDS population stratified by adherence to Mediterranean diet
During a mean follow-up period of 4.0 ± 1.5 years, incident cognitive impairment was identified in 1,248 individuals (7%). Incident stroke between the first and the last follow-up assessments was identified in 203 participants (1%). Results of incremental logistic regression models estimating odds ratios (ORs) for incident cognitive impairment after excluding cognitive assessments following incident stroke appear in table 2. High adherence to MeD was associated with lower likelihood of incident cognitive impairment in unadjusted logistic regression models (model I) and after adjusting for demographic characteristics (model II), environmental factors (model III), vascular risk factors and antihypertensive medications (model IV), depressive symptoms and self-reported health-status (model V). More specifically, in the final incremental logistic regression model adjusting for all potential confounders, high adherence to MeD was associated lower likelihood of incident cognitive impairment (OR 0.87; 95% confidence interval [CI] 0.76–1.00, p = 0.0460).
Table 2.
Association of adherence to Mediterranean diet with incident cognitive impairment on incremental logistic regression models (excluding cognitive assessments after incident stroke) (the MeD was dichotomized using a median split [0–4 vs 5–9])
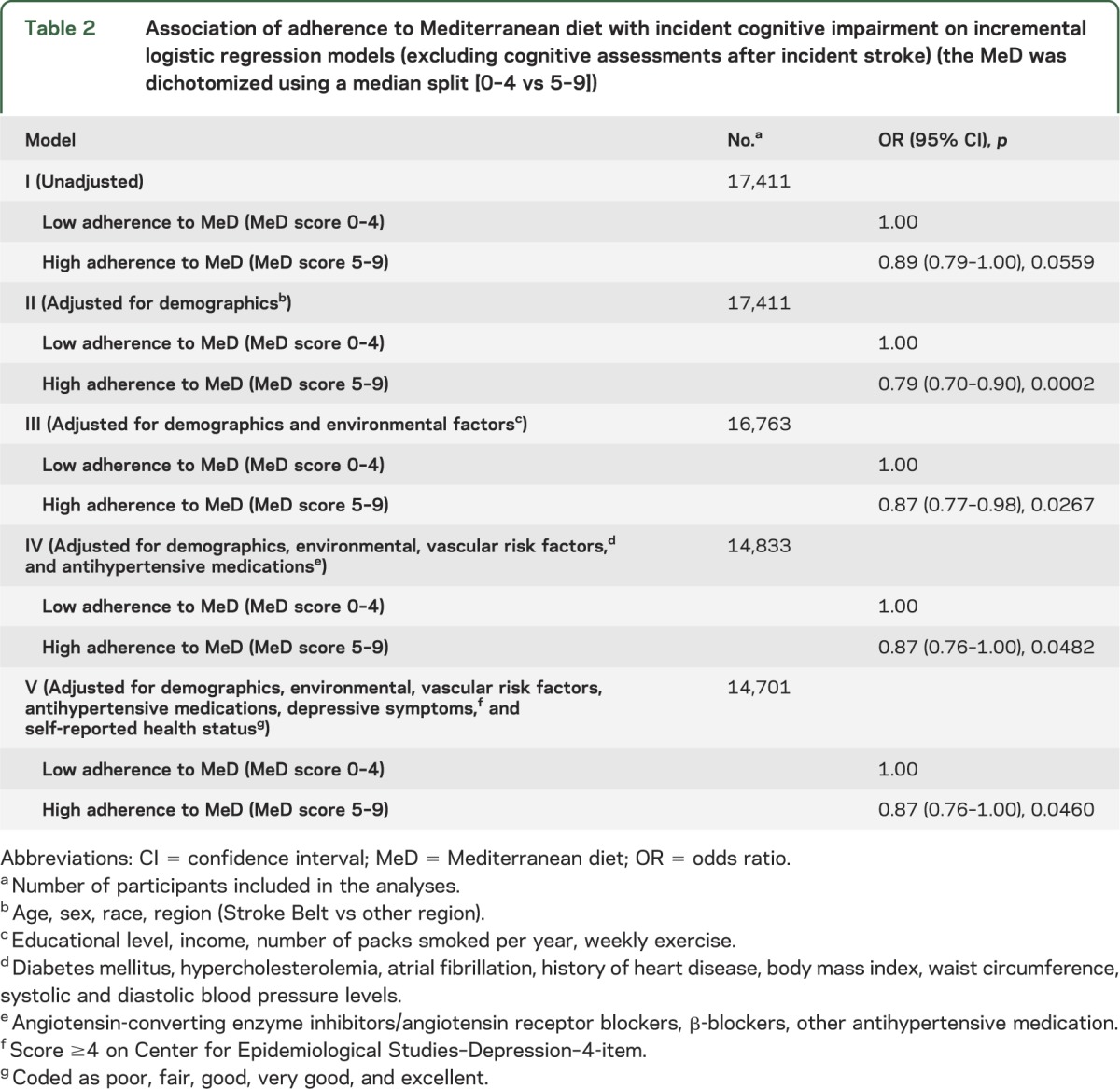
The relationships were attenuated slightly after including data from cognitive assessments following incident stroke in the incremental logistic regression models. In particular, high adherence to MeD diet was associated with lower likelihood of incident cognitive impairment after adjusting for demographic characteristics (model II; p = 0.0003) and environmental factors (model III; p = 0.0414), but the former relationship did not retain its statistical significance at the 0.05 level after further adjustment for vascular risk factors and antihypertensive medications (model IV; p = 0.0752), depressive symptoms and self-reported health status (model V; p = 0.0715), and incident stroke (model VI; OR = 0.89; 95% CI 0.77–1.01; p = 0.0744) in incremental logistic regression models.
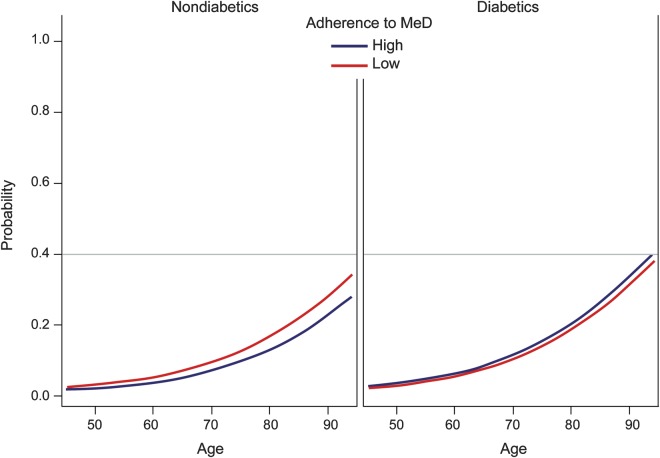
There was no evidence of interaction between race (p = 0.2928) or Stroke Belt region (p = 0.9978) and the association of adherence to MeD with incident cognitive impairment. However, we identified a strong interaction of DM (table 3, figure 2). More specifically, high adherence to MeD was associated with lower risk of incident cognitive impairment in the nondiabetic population but the former relationship was not significant in participants with DM (p for interaction 0.0110, 0.0138, and 0.0134 in models IV, V, and VI, respectively). In the final model adjusting for all potential confounders including incident stroke, high adherence to MeD was associated with lower likelihood of incident cognitive impairment (OR 0.81; 95% CI 0.70–0.94; p = 0.0066) in nondiabetic participants. In contrast, adherence to MeD was not related to impaired cognitive status among diabetic individuals (OR 1.27; 95% CI 0.95–1.71; p = 0.1063). We also assessed the interaction between other demographic characteristics or vascular risk factors and the association of adherence to MeD with incident cognitive impairment. We documented only one additional significant (p < 0.05) interaction. More specifically, the relationship of higher adherence of MeD with lower likelihood of incident cognitive impairment was mediated by age (p for interaction 0.021) in the model adjusting for demographic characteristics (model II). Participants with higher adherence to MeD tended to have lower odds of incident cognitive impairment at younger ages, but this effect disappeared at older ages (>80 years). The former interaction did not retain its statistical significance (p for interaction 0.140) in the final model adjusting for all potential confounders (model VI).
Table 3.
Interaction of diabetes mellitus on the relationship of adherence to Mediterranean diet with incident cognitive impairment
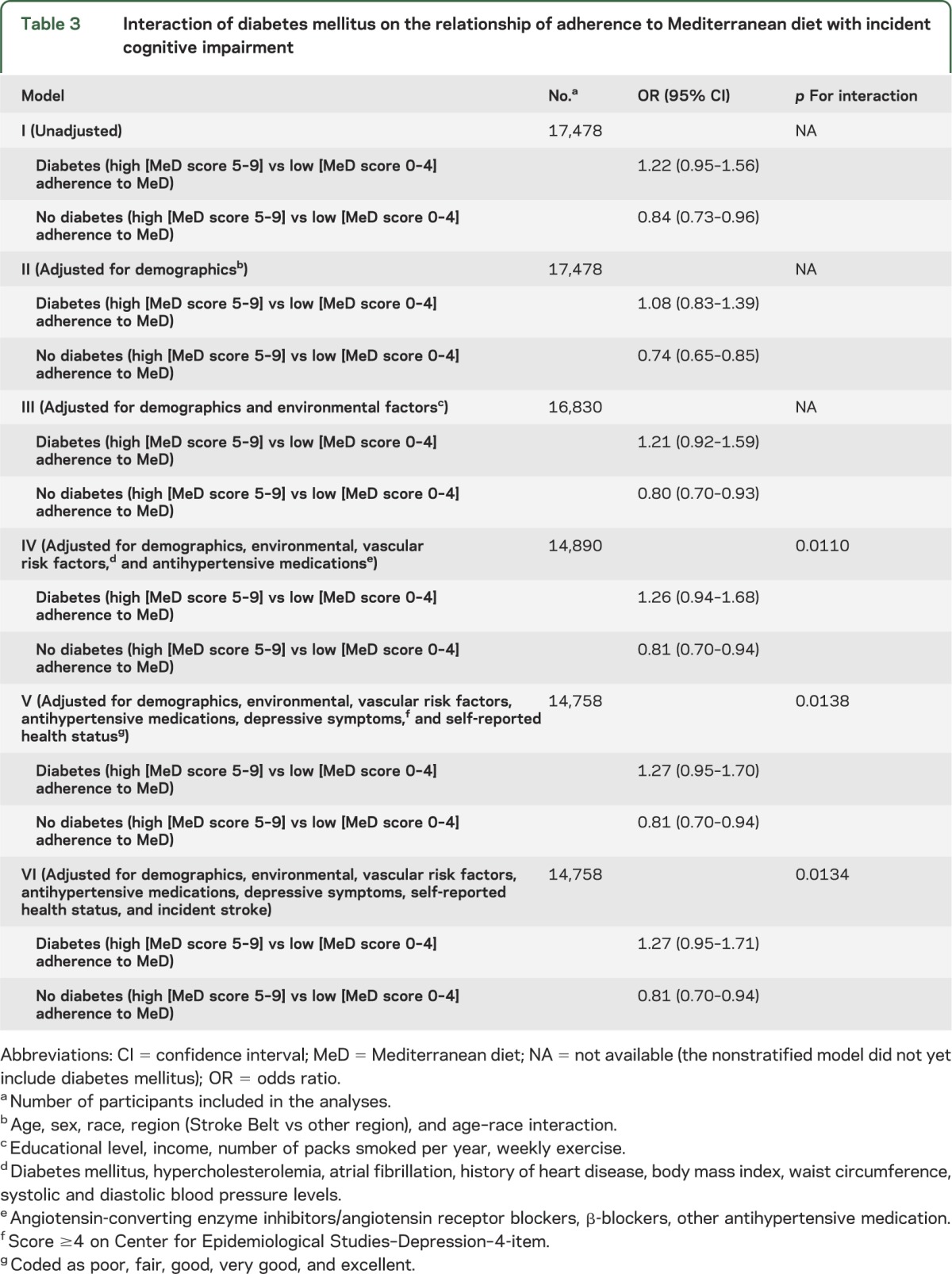
Figure 2. Interaction plot of diabetes mellitus and Mediterranean diet (MeD) adherence on the adjusted probability of cognitive decline.
We also repeated our analyses using tertiles to categorize adherence to MeD. In the final incremental logistic regression model adjusting for all potential confounders, adherence to MeD was associated with lower likelihood of incident cognitive impairment (p = 0.0034; p for linear trend = 0.0436). In addition, we performed the sensitivity analysis using a more stringent definition of incident cognitive impairment. The additional analyses yielded practically identical results for the unadjusted and fully adjusted models evaluating independent predictors of incident cognitive impairment in the study subsample of 15,963 individuals having at least 3 assessments (data not shown). Finally, we evaluated the association of adherence to MeD with cognitive function using mixed effect models in order to take into account SIS changes over time. In the full mixed effect model adjusting for all potential confounders including incident stroke, higher adherence to MeD was independently (p < 0.0001) associated with a higher SIS score.
DISCUSSION
Our longitudinal study showed that high adherence to MeD was associated with a lower likelihood of incident cognitive impairment in a large population-based sample of US black and white adults during a mean follow-up period of 4 years. This relationship persisted after adjustment for numerous potential confounders. Moreover, we found no evidence for interaction between race or region of residence and the relationship of adherence to MeD and incident impaired cognitive status. Finally, we documented a strong interaction of DM on the relationship of adherence to MeD with incident cognitive impairment with high adherence to MeD being associated with lower likelihood of incident impaired cognitive status in nondiabetic individuals but not in diabetic participants.
Our findings partly support the association of higher adherence to MeD and lower risk of incident dementia that was originally reported in a Northern Manhattan population-based cohort.15–17 Our results are also in line with a recent report indicating that adherence to an empirically derived healthy dietary pattern in middle life may help preserve global cognitive function and verbal memory in particular in middle-aged individuals.32 To our knowledge, this is the largest (exceeding 17,000 individuals) population-based sample of stroke-free (at baseline) individuals in whom higher adherence to MeD was associated with a lower likelihood of incident cognitive impairment independent of a substantial number of important potential confounders at baseline assessment. Notably, a French study that also recently attempted to reproduce this relationship in a population-based sample from Bordeaux documented only an association between higher adherence to MeD and slower Mini-Mental State Examination decline.18 However, adherence to MeD was not related to incident dementia in the French cohort.18 Despite the fact that the mean MeD score in our report (4.4 points) was practically identical to the scores documented by the North American (4.3 points)15 and French (4.4 points)18 reports, the following methodologic differences need to be taken into account when interpreting the discrepant findings across the studies: differences in sample size (ranging from 1,39317 to 2,25815 in the American vs 1,410 individuals18 in the French cohort), duration of the mean follow-up period (range 4.015 to 5.416 years in the US study vs 4.1 years18 in the French study), different cutoffs for the tertile split of MeD score, assessment of cognitive function using different neuropsychological measures, and country-specific characteristics of the dietary patterns. Moreover, the US studies did not adjust for depressive symptoms15,17 and previous stroke15–17 in contrast to the French report,18 where adjustment was performed for these important confounders.
Our findings did not confirm our prespecified hypothesis that the protective effect of MeD in decreasing the likelihood of incident cognitive impairment may be accentuated in black in comparison to white individuals. The former result may be partly explained by the finding that the typical dietary patterns in black subjects differ more from the MeD than the patterns in the general population. In particular, racial disparities in fruit and vegetable intake have been documented among white, black, and Hispanic individuals, with higher consumption of fruits and vegetables documented in white individuals.23,24 Also, the higher prevalence of DM in black subjects may have accounted for the lack of racial disparities on the association of higher adherence to MeD with increased likelihood of incident cognitive impairment, since adherence to MeD was not protective of incident impaired cognitive status in diabetic patients.
The interaction of DM on the association of higher adherence to MeD with incident cognitive impairment is intriguing. This observation may be partly explained by recent evidence indicating additional noncerebrovascular mechanisms linking DM to dementia including reduced amyloid clearance in the brain due to peripheral hyperinsulinemia33 and upregulation of receptor for advanced glycation end-products ligands in diabetic patients that have been shown to cause amyloid-β peptide neurotoxicity in AD models.34 Moreover, repeat episodes of hypoglycemia (a well-known complication of glucose-lowering therapies) have been proposed as an alternative noncerebrovascular mechanism linked to cognitive decline among diabetic individuals.35 Another plausible explanation may be attributed to the different dietary patterns of persons with DM (advocated macronutrients: carbohydrates 45%–60%, protein 15%–20%, and fat up to 35% of total daily energy)36 compared to nondiabetic individuals.
The findings of the present study are subject to certain limitations. For one, the lack of imaging data confines the ability to link adherence to MeD to neuropathology and incident cognitive deficits. Second, impaired cognitive status was evaluated using a global cognitive screener (SIS) that is less sensitive and specific to vascular cognitive impairment and subtle cognitive changes than more fine-grained measures. In addition, SIS cannot reliably differentiate mild cognitive impairment from dementia. We also acknowledge that using cutpoints to designate categorical outcomes has inherent limitations, and that ambiguity surrounding interpretation of scores at the cutpoint of 4 vs 5 is inevitable. However, we believe that this approach is reasonable in a large sample similar to our dataset. Also, it should be kept in mind that the previously validated SIS28 has been extensively evaluated in the REGARDS dataset.31,37,38 More specifically, previous findings from REGARDS attest to the utility of SIS in detecting broad patterns of association with conditions affecting cognition including traditional cardiovascular risk factors37 and chronic kidney disease.38 Finally, the SIS has been used outside the REGARDS dataset to document cognitive impairment in older patients seen in emergency departments39 and older depressed patients in a large randomized controlled trial.40
Limitations related to the construction of Mediterranean-type diet score (equal weighting of underlying food categories and underestimation of total food and caloric intake) also need to be taken into account. Moreover, the assessment of dietary intake was performed only at baseline and thus we were unable to capture potential changes in dietary patterns during the follow-up period. Additionally, it is possible that selection or recall biases may be present in those returning the FFQ, since the dietary assessment was self-administered and subject to differential return by participants. However, it should be kept in mind that the return rate in REGARDS was approximately 70%, which is much higher than any other self-administered questionnaire return rate in other similar large, population-based cohort studies. Consequently, any potential bias introduced by differences between individuals returning and not completing the FFQ should be relatively minor given the high return rate achieved in our cohort. Nongeneralizability of the MeD score as a tool to assess dietary pattern, due to the already mentioned cross-country food habit differences, should also be acknowledged as a potential methodologic shortcoming of the present report. Finally, the observational design of the present study cannot rule out the possibility of residual confounding by unknown risk factors including genetic predispositions (APOE ε genotype), functional capacity (ability to perform activities of daily living or instrumental activities of daily living), living arrangements (home-dwelling or institutionalized), and higher adherence to medication intake of individuals adhering to a MeD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their contributions. A full list of participating REGARDS investigators and institutions is available online.
GLOSSARY:
- AD
Alzheimer disease
- BMI
body mass index
- CI
confidence interval
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- FFQ
Food Frequency Questionnaire
- MeD
Mediterranean diet
- OR
odds ratio
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- SBP
systolic blood pressure
- SIS
Six-item Screener
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Georgios Tsivgoulis had the idea for the article and data analyses and wrote the first version of the manuscript. Suzanne Judd contributed to the statistical analyses and editing of the manuscript. Abraham J. Letter contributed to the analysis plan, analyzed the data, and contributed to editing of the manuscript. Andrei V. Alexandrov contributed to editing of the manuscript. George Howard contributed to editing of the manuscript. Fadi Nahab contributed to editing of the manuscript. Frederick W. Unverzagt contributed to the study design and editing of the manuscript. Claudia Moy contributed to editing of the manuscript. Virginia J. Howard contributed to editing of the manuscript. Brett Kissela contributed to the study design and editing of the manuscript. Virginia G. Wadley contributed to the study design and analysis plan and editing of the manuscript.
STUDY FUNDING
Supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, NIH, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views and positions of the National Institute of Neurological Disorders and Stroke or the NIH. Representatives of the funding agency were involved in the review of the manuscript prior to submission for publication. G. Tsivgoulis has been supported by European Regional Development Fund Project FNUSA-ICRC (no. CZ.1.05/1.1.00/02.0123).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabbagh MN. Drug development for Alzheimer's disease: where are we now and where are we headed? Am J Geriatr Pharmacother 2009;7:167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol 2004;3:579–587 [DOI] [PubMed] [Google Scholar]
- 4.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer’s disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 2004;61:82–88 [DOI] [PubMed] [Google Scholar]
- 5.Morris MC, Evans DA, Bienias JL, Tagney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol 2002;59:1125–1132 [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 1998;55:1449–1455 [DOI] [PubMed] [Google Scholar]
- 7.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 1997;145:33–41 [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–946 [DOI] [PubMed] [Google Scholar]
- 9.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer's disease. Curr Neurol Neurosci Rep 2007;7:366–372 [DOI] [PubMed] [Google Scholar]
- 10.Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet 2002;359:281–286 [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulos D, Lagiou P. Mediterranean diet and cardiovascular epidemiology. Eur J Epidemiol 2004;19:7–8 [DOI] [PubMed] [Google Scholar]
- 12.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–2608 [DOI] [PubMed] [Google Scholar]
- 13.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomark Prev 2000;9:869–873 [PubMed] [Google Scholar]
- 15.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 2006;59:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009;66:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Féart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology 1997;49:44–50 [DOI] [PubMed] [Google Scholar]
- 20.Heyman A, Fillenbaum G, Prosnitz B, Raiford K, Burchett B, Clark C. Estimated prevalence of dementia among elderly black and white community residents. Arch Neurol 1991;48:594–598 [DOI] [PubMed] [Google Scholar]
- 21.Fillenbaum GG, Heyman A, Huber MS, et al. : The prevalence and 3-year incidence of dementia in older black and white community residents. J Clin Epidemiol 1998;51:587–595 [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Differences in prevalence of obesity among black, white, and Hispanic adults: United States, 2006–2008. MMWR Morb Mortal Wkly Rep 2009;58:740–744 [PubMed] [Google Scholar]
- 23.Dubowitz T, Heron M, Bird CE, et al. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am J Clin Nutr 2008;87:1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz VA, Mainous AG, 3rd, Koopman RJ, Carek PJ, Geesey ME. Race and diet in the overweight: association with cardiovascular risk in a nationally representative sample. Nutrition 2005;21:718–725 [DOI] [PubMed] [Google Scholar]
- 25.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 26.Wadley VG, McClure LA, Howard VJ, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke 2007;38:1143–1147 [DOI] [PubMed] [Google Scholar]
- 27.Tsivgoulis G, Alexandrov AV, Wadley VG, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009;73:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item Screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781 [DOI] [PubMed] [Google Scholar]
- 29.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469 [DOI] [PubMed] [Google Scholar]
- 30.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas 1993;53:1117–1125 [Google Scholar]
- 31.Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the Stroke Belt: the REGARDS study. Ann Neurol 2011;70:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr 2012;142:909–915 [DOI] [PubMed] [Google Scholar]
- 33.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–152 [DOI] [PubMed] [Google Scholar]
- 34.Yan SF, Yan SD, Ramasamy R, Schmidt AM. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med 2009;41:408–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmer RA, Karter AJ, Yaffe K, Quesenberry J, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Diabetes Association Nutrition recommendations and interventions for diabetes. Diabetes Care 2008;31:S61–S78 [DOI] [PubMed] [Google Scholar]
- 37.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011;77:1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008;52:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med 2005;12:612–616 [DOI] [PubMed] [Google Scholar]
- 40.Steffens DC, Snowden M, Fan MY, Hendrie H, Katon WJ, Unutzer J. Cognitive impairment and depression outcomes in the IMPACT study. Am J Geriatr Psychiatry 2006;14:401–409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.