Abstract
Objective:
In an effort to account for deficiencies in axonal transport that limit the effectiveness of neurotrophic factors, this study tested the safety and feasibility, in moderately advanced Parkinson disease (PD), of bilaterally administering the gene therapy vector AAV2-neurturin (CERE-120) to the putamen plus substantia nigra (SN, a relatively small structure deep within the midbrain, in proximity to critical neuronal and vascular structures).
Methods:
After planning and minimizing risks of stereotactically targeting the SN, an open-label, dose-escalation safety trial was initiated in 6 subjects with PD who received bilateral stereotactic injections of CERE-120 into the SN and putamen.
Results:
Two-year safety data for all subjects suggest the procedures were well-tolerated, with no serious adverse events. All adverse events and complications were expected for patients with PD undergoing stereotactic brain surgery.
Conclusions:
Bilateral stereotactic administration of CERE-120 to the SN plus putamen in PD is feasible and this evaluation provides initial empirical support that it is safe and well-tolerated.
Classification of evidence:
This study provides Class IV evidence that bilateral neurturin gene delivery (CERE-120) to the SN plus putamen in patients with moderately advanced PD is feasible and safe.
Abundant preclinical research argues that neurotrophic factors offer promising therapies for Parkinson disease (PD).1 Clinical trials have generally been disappointing, likely because of difficulties delivering sustained bioactive protein throughout the targeted brain region. Gene transfer can potentially solve these problems.1 A double-blind study testing putaminal gene delivery of the neurotrophic factor neurturin (NRTN) failed to meet its primary endpoint at 12 months but showed benefit for the predefined primary endpoint at 18 months and for several secondary endpoints at 12 and 18 months (no measure similarly favored sham).1,2 Postmortem studies suggest that serious deficiencies in axonal transport in PD limited NRTN transport to the substantia nigra pars compacta (SNc), thereby impairing the ability of putaminal delivery to activate repair genes in SNc neurons, thereby reducing and delaying clinical benefit.3 We therefore initiated a study testing NRTN gene delivery to both SNc and putamen. However, a number of safety concerns have been raised regarding stereotactically targeting the SNc and expressing neurotrophic factors in the substantia nigra (SN) and surrounding structures.4–6 Based on safety associated with targeting the SNc and neighboring regions in patients with PD with deep brain stimulation7 and fetal tissue transplant,8 and the lack of serious side effects observed following NRTN gene delivery to the SNc in preclinical studies,9 we began a clinical trial in patients with advanced PD. In the present article, we present the 24-month results of an open-label safety study of 6 patients who underwent bilateral NRTN gene delivery (CERE-120) to the putamen and SNc.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study is registered with ClinicalTrials.gov, number NCT00985517, was approved by the US Food and Drug Administration, and was publicly discussed and reviewed by the Recombinant DNA Advisory Committee of the NIH. The study protocol and patient consent forms were approved by institutional ethics and biological safety committees at each institution. Each patient signed informed consent before entry into the trial.
Subjects.
Inclusion/exclusion criteria were similar to prior AAV2-NRTN clinical trials, involving subjects diagnosed with idiopathic PD according to UK Brain Bank criteria.2,10
Dosing and assessment.
To assess the feasibility and safety of targeting the SN plus putamen with AAV2-NRTN in PD, 2 dose cohorts were tested (3 subjects each) with a 1-month interval between each surgery and a 5-week hiatus between the low-dose and high-dose cohorts. An independent data monitoring committee (DMC) reviewed the entire CERE-120 safety profile after each surgery. In the first cohort, patients received a total dose of CERE-120 of 4.0 × 1011 vector genomes (vg) to the SN, and 5.4 ×1011 vg to the putamen (the same putaminal dose used in the prior phase IIa CERE-120 trial).2 The SN dose was determined based on between-species “dose scaling,” designed to adequately cover the SN without risk of substantial spread to non-SN structures.9 The second dose cohort received the same dose to the SN and a nearly 4-fold higher dose to the putamen, for a total dose of 24.0 × 1011 vg, in a volume of 360 μL. The infusion rate was increased from 2 μL/min to 3 μL/min for the putamen (but remained 2 μL/min for the SN), using an automated infusion pump (World Precision Instruments, Sarasota, FL).
Following surgery, subjects were evaluated for safety and tolerability at week 2, month 1, month 3, and every 3 months thereafter for the next 2 years, as well as on number of motor and quality-of-life measurements assessed at baseline and at visits starting at month 6. This design, wherein each subject served as his or her own control (and comparisons in performance were made on protocol-prescribed, objective measures between pretreatment baseline scores and a series of post-treatment scores), was intended to provide Class IV evidence regarding the feasibility and safety of administering CERE-120 to the SN and putamen in patients with moderately advanced PD.
Stereotactic targeting and surgery.
Targeting and stereotactic surgery were performed in a similar manner to past gene transfer of NRTN studies for PD.2,10 All subjects were administered CERE-120 bilaterally, using a single burr hole per hemisphere. To help ensure uniformity of dosing across subjects and clinical sites, and to reduce the risk of mistargeted protein, a consensus with respect to targets and trajectories was required between the operating neurosurgeon and an independent neurosurgical reviewer.
Two CERE-120 bolus infusions were delivered to the SN via one trajectory in each hemisphere; doses were separated by 2–4 mm (figure). CERE-120 was then delivered to the putamen via 3 trajectories per hemisphere. The 3 putaminal target sites were evenly spaced across the anterior-posterior extent of the putamen. If an extremely narrow area at the posterior end of the putamen occurred, this “tail” was excluded for purposes of selecting target locations. In each subject, 2 of the 3 target locations were positioned posterior to the anterior commissure. All 3 targets were positioned in the ventral half of the putamen, approximately two-thirds of the way down from the dorsalmost edge, but at least 4 mm from the inferior boundary, and all were relatively centered in the medial-lateral dimension.
Figure. Artist's rendition of the dosing scheme employed to bilaterally target the substantia nigra and putamen with AAV2-NRTN (CERE-120).
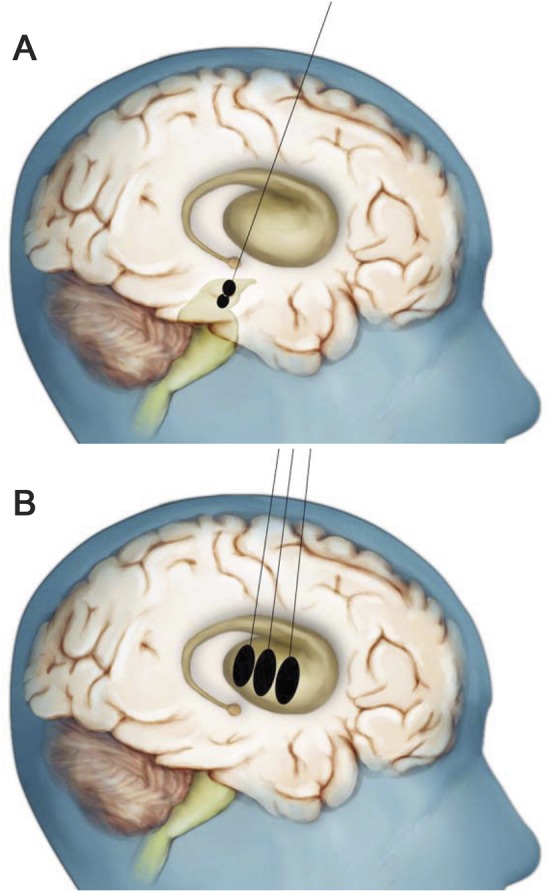
(A) A single needle tract was used to deliver 2 infusions into each substantia nigra (SN). (B) Three additional needle tracts were used to deliver 3 equally spaced infusions into each putamen. Note that only a single burr hole was required to accommodate the 4 needle tracts per each hemisphere. The initial, low-dose cohort received the same dose of CERE-120 into the SN as the high-dose cohort, but only about one-fourth the putaminal dose. See text for additional details. This figure is a variation of one previously published in “Translating the therapeutic potential of neurotrophic factors to clinical ‘proof of concept’: a personal saga achieving a career-long quest”13 and in “Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating ‘clinical proof-of-concept’ for AAV-neurturin (CERE-120) in Parkinson's disease,”1 © Elsevier (2012) with permission.
Postsurgical MRIs were reviewed by a central neuroradiologist (Daniel Lefton, Beth Israel Medical Center, New York, NY) to confirm adherence to targeting guidelines and to identify any safety issues. In many instances a clear signal from the needle track could be seen on the postsurgical MRI confirming appropriate trajectories to the putamen or SN, though it was never possible to identify with certainty the location of the tip of the injection cannula. In no case was any evidence for any mistargeted trajectories observed, nor did any individual differences in size or shape of putamen negatively impact the ability to target it.
RESULTS
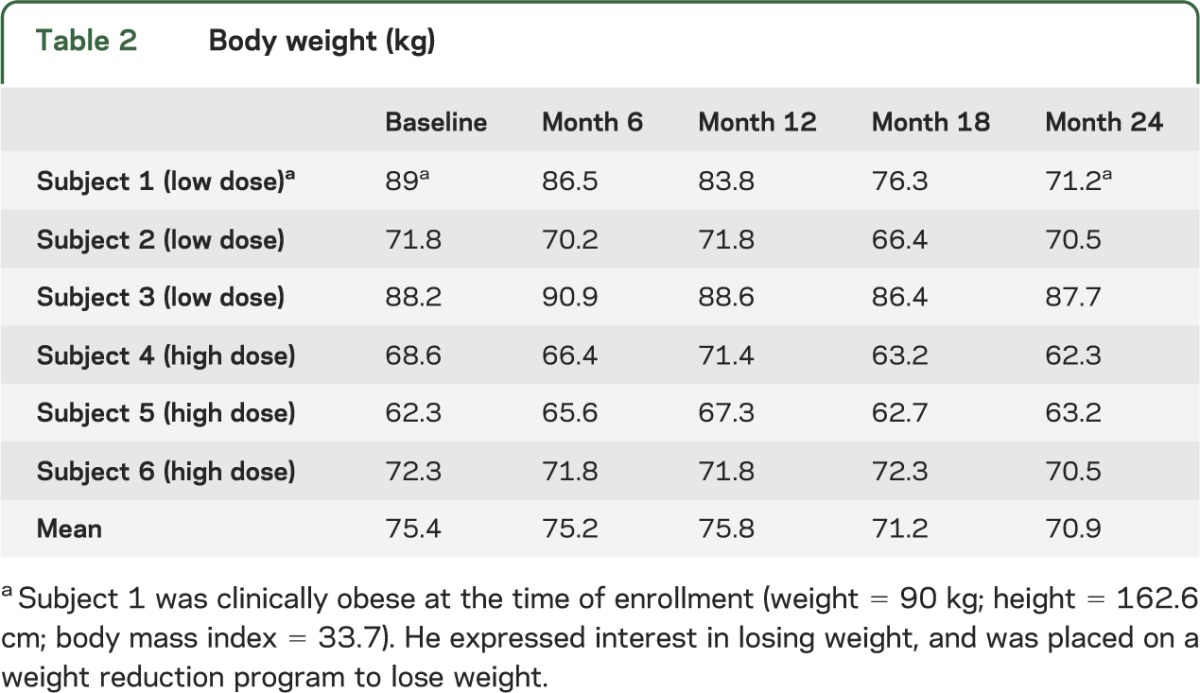
Six subjects underwent the procedure and were followed for 2 years. Demographic information is provided in table 1. No serious adverse events (SAEs) were reported. The most common adverse events were incision site pain (n = 4), dyskinesias (n = 4), headache (n = 4), and abnormal dreams (n = 4); these were transient, considered clinically insignificant, and unrelated to AAV2-NRTN treatment. Dyskinesias reported in this study were not increased in severity, occurred no more frequently than in the earlier putaminal-only phase I CERE-120 study, and were all transient.10 No patient experienced off-medication dyskinesia. No clinically significant changes were detected on physical or neurologic examination or vital signs. Specifically, there was no clinically significant weight loss associated with the procedure (table 2) and no subject experienced psychosis or clinically significant alteration in mental status. Laboratory tests of serum revealed no abnormalities and no increase in AAV antibodies, detectable NRTN antibodies, or CERE-120 vg. MRIs of the brain at day 1 and months 1, 12, and 24 postoperatively revealed no clinically significant abnormalities related to either the surgical procedure or CERE-120. An independent DMC had access to all data and had no safety concerns. The Unified Parkinson's Disease Rating Scale, motor-off, suggested a decrease from 38.2 (±2.6 SEM) to 33.2 (±3.5) and 32.7 (±3.6) at 12 and 24 months, respectively, and a decrease in off-time on self-report home diary measures from 5.4 (±0.8) hours at baseline to 4.3 (±1.5) and 3.1 (±0.7) at 12 and 24 months, respectively. Parkinson's Disease Questionnaire-39: Activities of Daily Living scores appeared stable (25 ± 2.6 and 25 ± 6.1 at baseline and 24 months, respectively).
Table 1.
Characteristics of patientsa
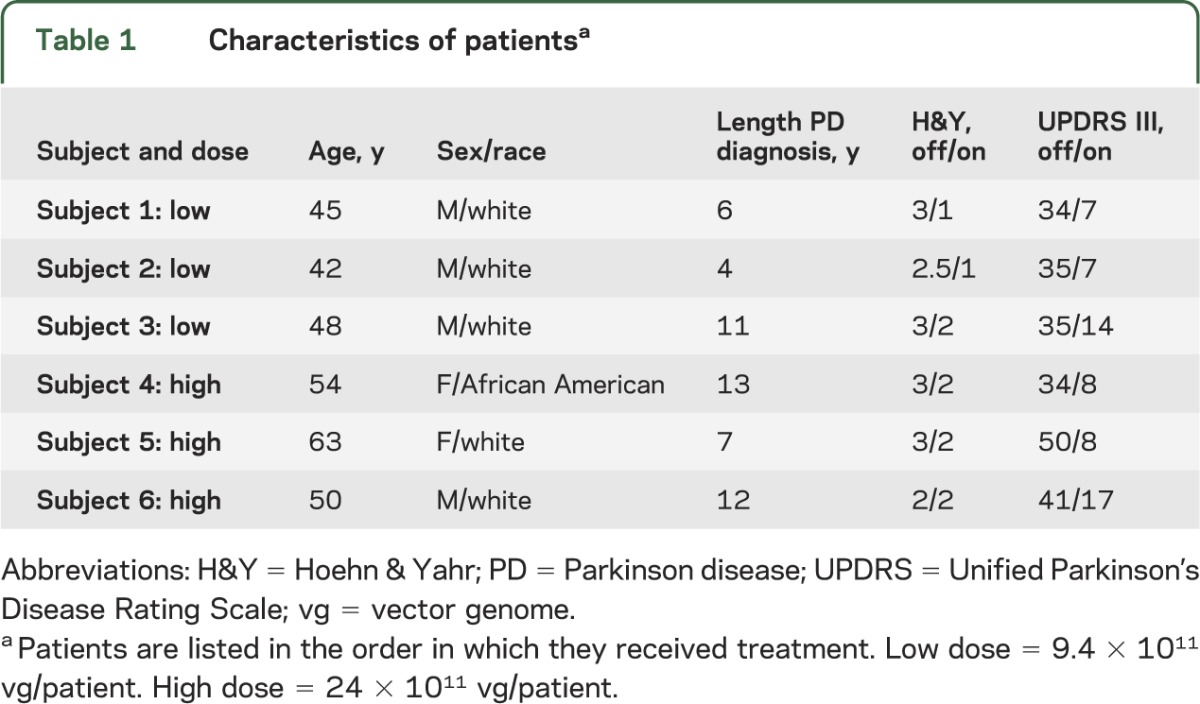
Table 2.
Body weight (kg)
DISCUSSION
We report here the long-term (2-year) safety results from 6 subjects with PD who were treated with AAV2-NRTN (a gene-transfer viral vector encoding the neurotrophic factor NRTN) delivered to both the SNc and putamen. In this phase I study, CERE-120 delivered into both the SNc and putamen was well-tolerated, with no safety or serum laboratory complications identified over the course of 2 years of follow-up. No SAEs were reported in any subject; all adverse events were expected for an advanced PD patient population undergoing a neurosurgical procedure, and none was clinically significant or enduring. Specifically, no problems were encountered with respect to stereotactically targeting the SNc, expression of a trophic factor in the SNc, or possible spread to adjacent sites like the ventral tegmental area or hypothalamus. More specifically, we did not observe weight loss, which has been reported as a potential concern for targeting the SN with neurotrophic factors, based on certain preclinical studies,5,6,11 though others suggested that outcome is likely due to mistargeting protein extending into the ventricles or hypothalamus.9 We also did not observe any evidence of psychiatric symptoms such as addiction or psychosis that theoretically might result from trophic enhancement of the ventral tegmental area.4 Thus, these data provide support for the feasibility and safety of targeting both the SN and putamen with AAV2-NRTN in subjects with moderately advanced PD.
This open-label study was designed to generate preliminary safety and tolerability information. Based on the data generated in this study, a multicenter, double-blind, sham surgery–controlled trial has been launched to assess the effect of combined intraputaminal and SNc gene delivery of NRTN on key motor and quality-of-life measurements in 51 patients with moderately advanced PD. Consistent with the 2-year safety results reported here, no serious, unexpected safety issues have been encountered after more than a year of follow-up after dosing in any of the subjects in this ongoing phase IIb study.12
ACKNOWLEDGMENT
The authors thank Daniel Lefton and David Barba for help and advice regarding stereotactically targeting the SNc; Eugene Johnson, Jeffrey Kordower, Floyd Bloom, Rusty Gage, Karl Kieburtz, Ray Watts, Barry Hoffer, Paul Larson, Herbert Meltzer, and Carol Tamminga for comments and advice during due diligence of assessing the risks vs benefits; Stewart Factor, Michele Tagliati, and Catherine Cho, who served as PIs for 2 sites and enrolled and assessed 2 of the 6 subjects in this phase I trial; the trial coordinators at each site who provided operational support; Jeffrey Ostrove for support throughout this study; Amanda Sánchez Losada for administrative assistance in helping to prepare this manuscript, including formatting of tables and references; and the Michael J. Fox Foundation for Parkinson's Research for assistance in the form of a LEAPS award to R.T.B. and J.S.
GLOSSARY:
- DMC
data monitoring committee
- NRTN
neurturin
- PD
Parkinson disease
- SAE
serious adverse event
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- vg
vector genomes
AUTHOR CONTRIBUTIONS
R.T. Bartus contributed to the design and concept of the study, analysis and interpretation of the data, and drafting and revising the manuscript for intellectual content. He also wrote the initial draft, along with C.W.O. T.L. Baumann contributed to the design and concept of the study, analysis and interpretation of the data, and drafting and revising the manuscript for intellectual content. J. Siffert contributed to the design and concept of the study, analysis and interpretation of the data, and drafting and revising the manuscript for intellectual content. C.D. Herzog contributed to the design of parts of the study, interpretation of the data, and revisions to the manuscript for intellectual content. R. Alterman contributed to the design of the surgical component of the study, analysis and interpretation of the data, and revising the manuscript for intellectual content. N. Boulis contributed to the design and concept of the surgical component of the study, interpretation of the data, and revisions to the manuscript for intellectual content. D.A. Turner contributed to the design of the surgical component of the study, interpretation of the data, and revisions to the manuscript for intellectual content. M. Stacy contributed to the design of the study, interpretation of the data, and drafting and revising the manuscript for intellectual content. A.E. Lang contributed to the design and concept of the study, interpretation of the data, and drafting and revising the manuscript for intellectual content. A.M. Lozano contributed to the design and concept of the surgical component of the study, analysis and interpretation of the data, and revisions to the manuscript for intellectual content. C.W. Olanow contributed to the design and concept of the study, analysis and interpretation of the data, and drafting and revising the manuscript for intellectual content. He also wrote the initial draft, along with R.T.B. He has accepted responsibility to be guarantor for this manuscript.
STUDY FUNDING
This study was funded by Ceregene, Inc., with partial funding received from the Michael J. Fox Foundation for Parkinson's Research via a competitive LEAPS award to R.T.B. and J.S.
DISCLOSURE
R.T. Bartus is an employee of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives salary, benefits, and stock options. He is receiving grant support from the MJFF. T.L. Baumann is an employee of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives salary, benefits, and stock options. J. Siffert is a former employee of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and had previously received salary and benefits and still has stock options. He is currently employed at Avanir Pharmaceuticals, Inc., and also received grant support from the MJFF. C.D. Herzog is an employee of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives salary, benefits, and stock options. R. Alterman was an investigator for this trial and a paid consultant prior to its launch. N. Boulis has been a consultant to Ceregene and currently consults for NeuralStem. D.A. Turner is a consultant for Medtronics and has grants from NIH, the Defense Department, and the VA. M. Stacy was an investigator for this trial and is a consultant for Allergan, Chelsea, GE, GlaxoSmithKline, Merck, Merz, Neuronova, Novartis, Noven, Osmotica, SK Life Sciences, TEVA, and UCB. He has received grant support from IMPAX, MJFF, NIH, Novartis, and the Parkinson's Study Group. A.E. Lang is a member of the Scientific Advisory Board of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives financial remuneration and stock options. He is also a paid advisor to Boehringer-Ingelheim, Cephalon, Eisai, Medtronic, Lundbeck A/S, NeuroMolecular, Novartis, Solvay, Taro, and Teva. Grants: Canadian Institutes of Health Research, Dystonia Medical Research Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Ontario Problem Gambling Research Centre, and Parkinson's Disease Foundation. Andres M. Lozano is a member of the Scientific Advisory Board of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives financial remuneration and stock options. He is also a member of the advisory boards for Johnson & Johnson (Corporate Office of Science and Technology), Codman, Brainstorm Cell Therapeutics, Neurophage, Aleva, and Alcyone Life Sciences. He is a cofounder and Chair of the SAB for Functional Neuromodulation and a consultant for Medtronic, St-Jude, Boston Scientific, Amgen, Ely Lilly, Bristol Myers, Elekta, Bayer, Schering-Plough, QIG, Focused Ultrasound Foundation, Neuronova, and Neurospace. C.W. Olanow is a member of the Scientific Advisory Board of Ceregene, which is developing AAV2-NRTN for Parkinson's disease, and receives financial remuneration and stock options. He also is a paid consultant for Novartis, Orion, Teva Pharmaceuticals, Lundbeck, Merck-Serono, and Abbott. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bartus RT, Baumann T, Brown L, Kruegel B, Ostrove JM, Herzog CD. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating ‘clinical proof-of-concept’ for AAV-neurturin (CERE-120) in Parkinson’s disease. Neurobiol Aging 2013;34:35–61 [DOI] [PubMed] [Google Scholar]
- 2.Marks WJ, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010;9:1164–1172 [DOI] [PubMed] [Google Scholar]
- 3.Bartus RT, Herzog CD, Chu Y, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov Disord 2011;26:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu L, Wang X, Wu P, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry 2009;66:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su X, Kells AP, Huang EJ, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther 2009;20:1627–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manfredsson FP, Tumer N, Erdos B, et al. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther 2009;17:980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord 2006;21(suppl 14):219–237 [DOI] [PubMed] [Google Scholar]
- 8.Mendez I, Dagher A, Hong M, et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study: report of three cases. J Neurosurg 2002;96:589–596 [DOI] [PubMed] [Google Scholar]
- 9.Bartus RT, Brown L, Wilson A, et al. Properly scaled and targeted AAV2-NRTN (neurturin) to the substantia nigra is safe, effective and causes no weight loss: support for nigral targeting in Parkinson's disease. Neurobiol Dis 2011;44:38–52 [DOI] [PubMed] [Google Scholar]
- 10.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol 2008;7:400–408 [DOI] [PubMed] [Google Scholar]
- 11.Manfredsson FP, Burger C, Rising AC, et al. Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector. Mol Ther 2009;17:1857–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann T, Lang AE, Lozano AM, Olanow CW, Bartus RT. AAV2-neurturin (CERE-120) and Parkinson's disease: the safety and feasibility of combined substantia nigral and putaminal stereotactic targeting via a Phase 1/2b clinical trial in advanced Parkinson's disease. Presented at the 16th International Congress of Parkinson’s Disease and Movement Disorders; June 20, 2012; Dublin
- 13.Bartus RT. Translating the therapeutic potential of neurotrophic factors to clinical ‘proof of concept': a personal saga achieving a career-long quest. Neurobiol Dis 2012;48:153–178 [DOI] [PubMed] [Google Scholar]


