Abstract
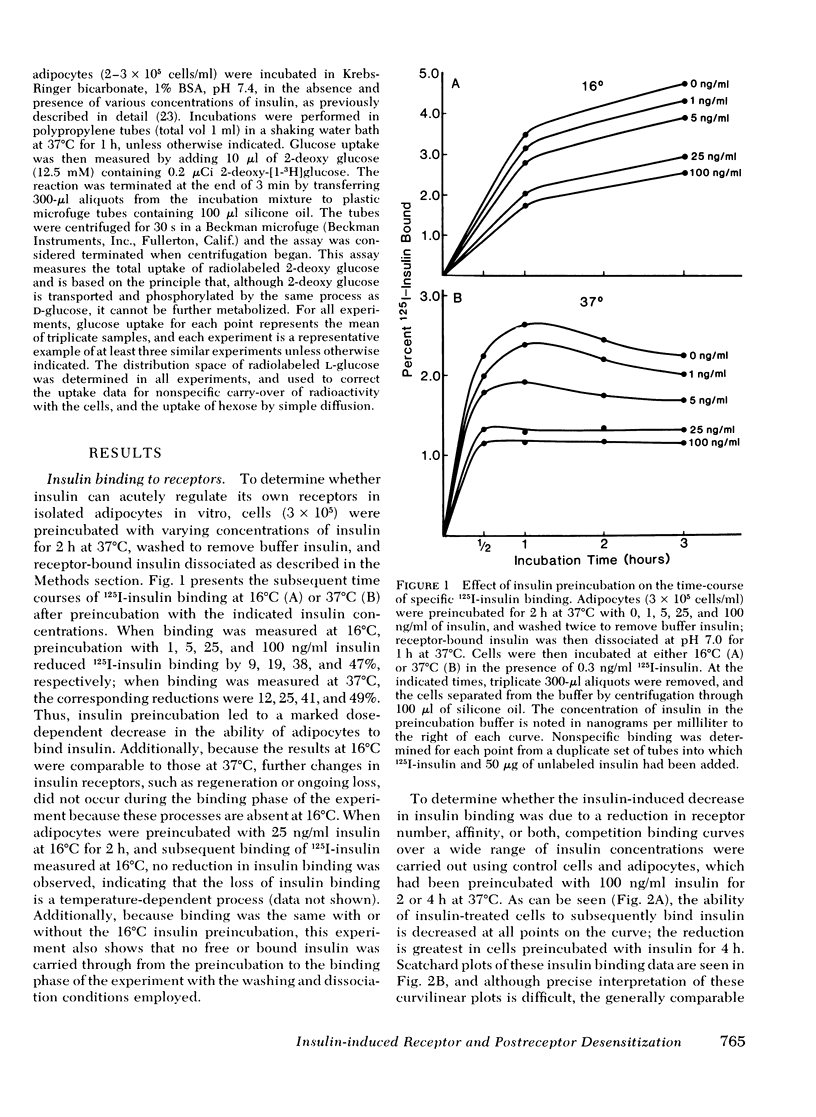
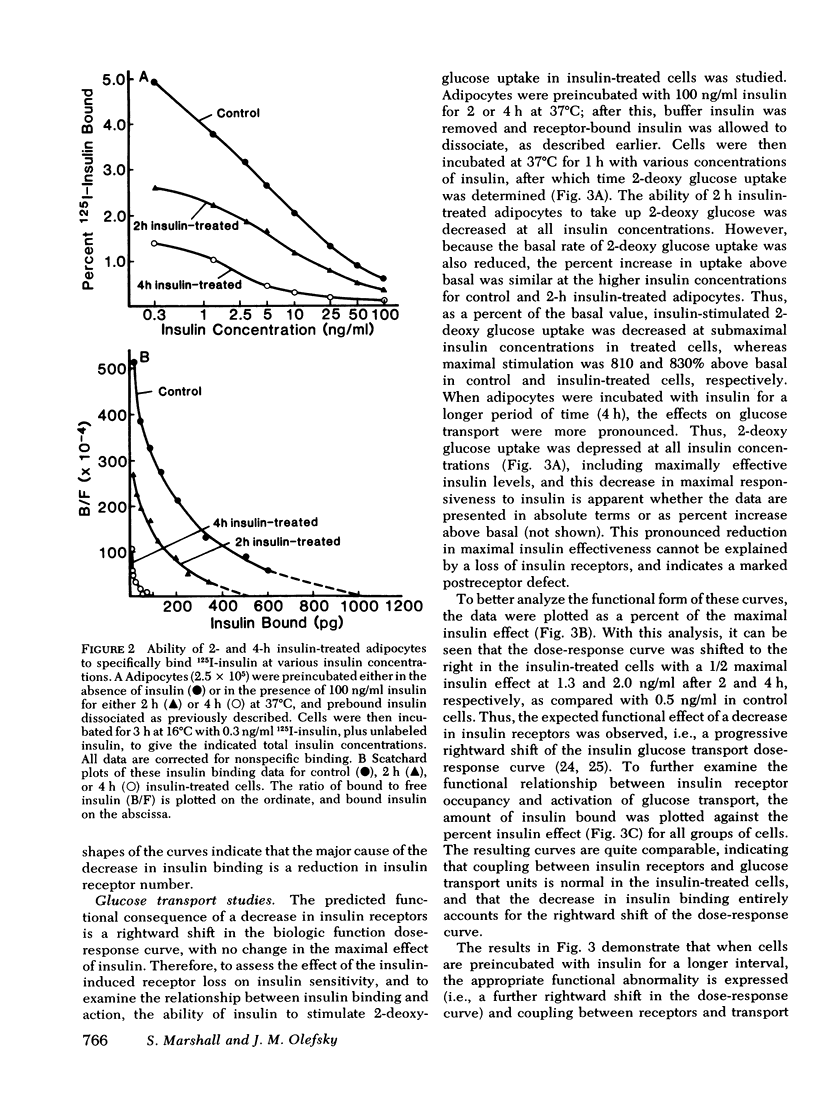
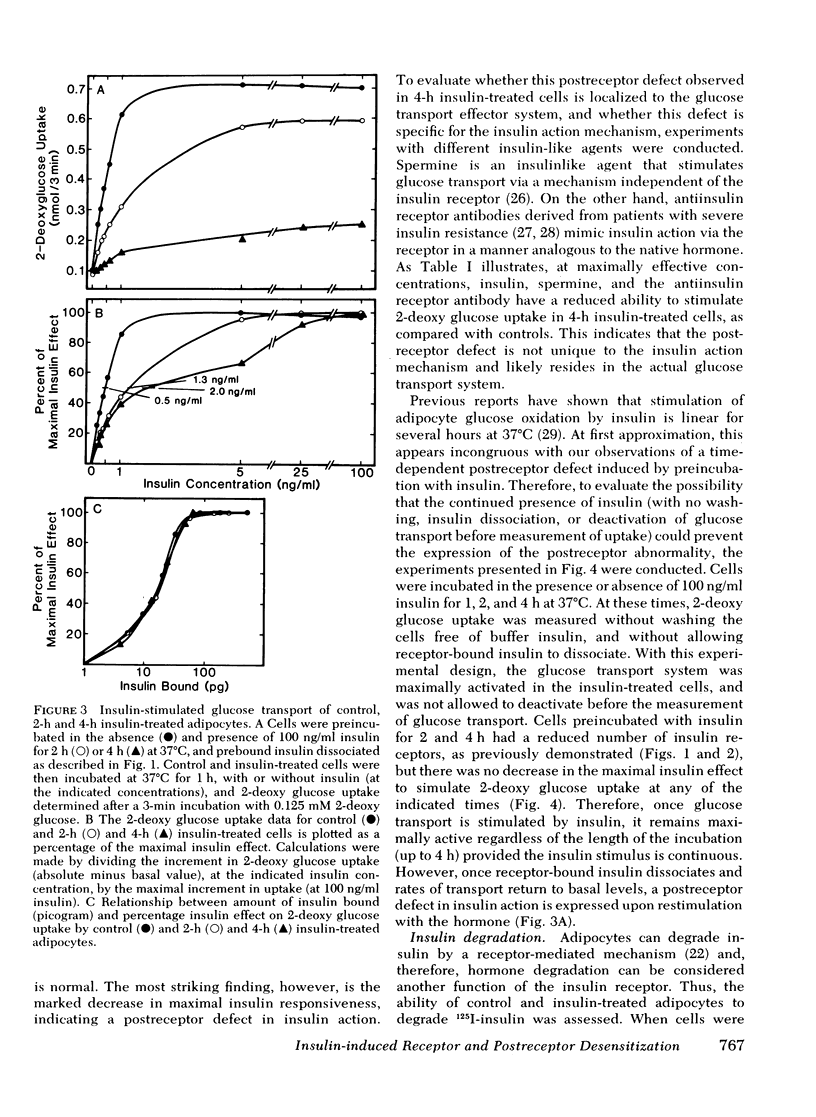
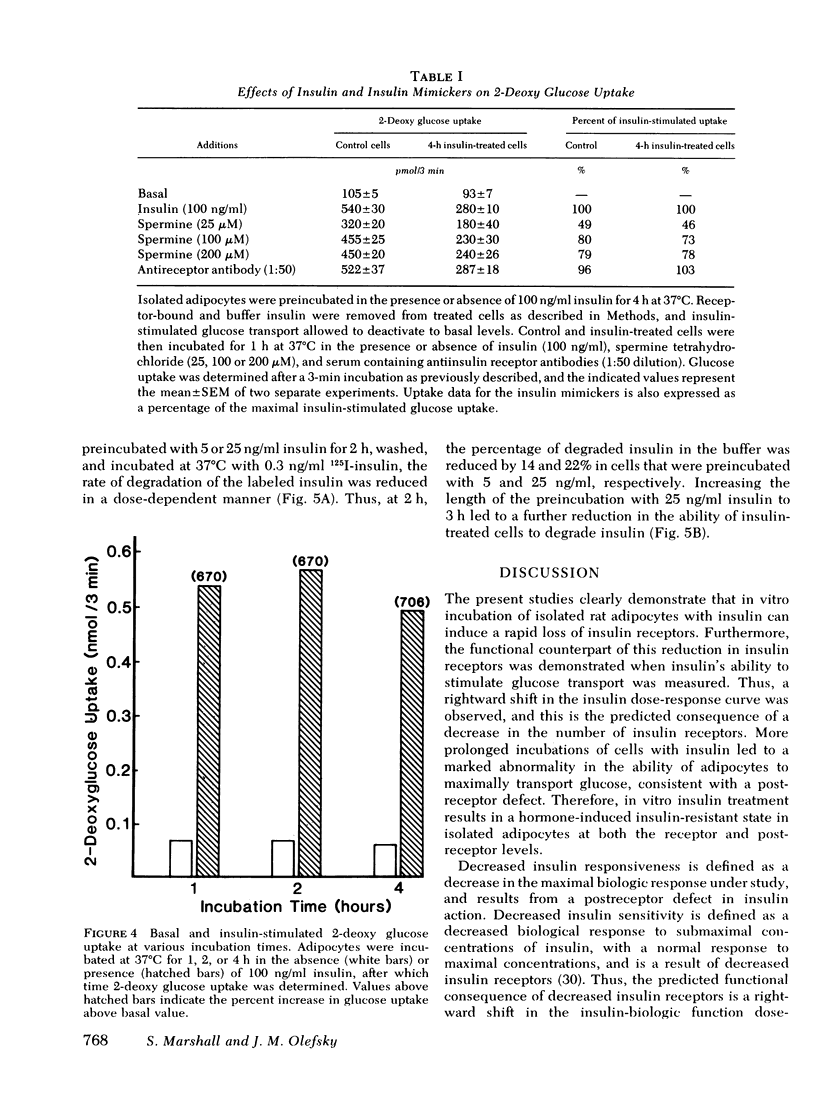
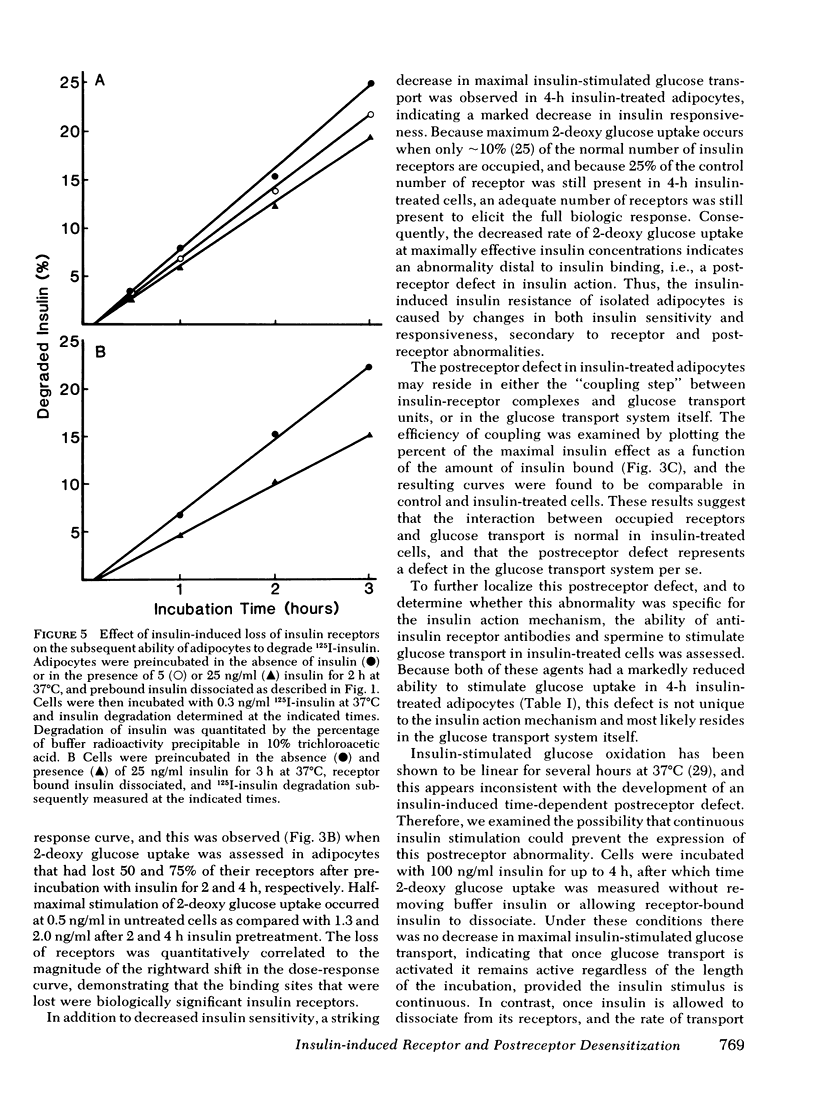
We have examined the effect of in vitro hyperinsulinemia on insulin binding, glucose transport, and insulin degradation in isolated rat adipocytes. When cells were incubated with insulin for 2 or 4 h at 37 degrees C, followed by washing in insulin-free buffer to remove extracellular and receptor-bound insulin, a time and dose-dependent decrease in insulin receptors was observed, which was accompanied by a reduced ability of cells to degrade insulin. Furthermore, the quantitatively predicted rightward shift in the insulin-glucose transport dose-response curve could be demonstrated. In addition to this reduction in insulin sensitivity, a striking decrease in maximal insulin-stimulated glucose transport was observed in the 4-h insulin-treated cells, indicating an abnormality distal to the insulin receptor. Thus, in vitro insulin-induced insulin resistance in adipocytes is caused by both receptor and postreceptor abnormalities. The post-receptor defect is most likely at the level of the glucose transport system per se because the insulinlike agents, spermine and antiinsulin receptor antibodies, also had a markedly reduced ability to stimulate glucose transport in 4-h insulin-treated cells. On the other hand, when cells were incubated with 100 ng/ml insulin for up to 4 h, after which time 2-deoxy glucose uptake was measured without removing buffer insulin or allowing receptor-bound insulin to dissociate, no decrease in maximal insulin-stimulated glucose transport was found. In conclusion, (a) insulin leads to a dose-dependent loss of insulin receptors in freshly isolated adipocytes accompanied by the predicted functional consequence of decreased receptors, i.e., a rightward shift in the insulin-glucose transport dose-response curve, (b) prolonged incubation with insulin causes a marked postreceptor defect in the glucose transport system, (c) maintenance of the activated state of the glucose transport system prevents the expression of the post-receptor defect, (d) the location of the postreceptor abnormality is most likely in the glucose transport system per se, and (e) insulin-induced receptor loss is accompanied by a decrease in insulin degradation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Guzelian P. S., Small M. E. Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology. 1978 Aug;103(2):548–553. doi: 10.1210/endo-103-2-548. [DOI] [PubMed] [Google Scholar]
- Chandramouli V., Milligan M., Carter J. R., Jr Insulin stimulation of glucose transport in adipose cells. An energy-dependent process. Biochemistry. 1977 Mar 22;16(6):1151–1158. doi: 10.1021/bi00625a019. [DOI] [PubMed] [Google Scholar]
- Chang T. H., Polakis S. E. Differentiation of 3T3-L1 fibroblasts to adipocytes. Effect of insulin and indomethacin on the levels of insulin receptors. J Biol Chem. 1978 Jul 10;253(13):4693–4696. [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J. Receptors, antireceptor antibodies and mechanisms of insulin resistance. N Engl J Med. 1979 Feb 22;300(8):413–419. doi: 10.1056/NEJM197902223000808. [DOI] [PubMed] [Google Scholar]
- Freychet P., Laudat M. H., Laudat P., Rosselin G., Kahn C. R., Gorden P., Roth J. Impairment of insulin binding to the fat cell plasma membrane in the obese hyperglycemic mouse. FEBS Lett. 1972 Sep 15;25(2):339–342. doi: 10.1016/0014-5793(72)80519-2. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effect of experimental hyperinsulinaemia on intracellular glucose metabolism of isolated adipocytes. Diabetologia. 1979 Aug;17(2):111–116. doi: 10.1007/BF01222211. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am J Physiol. 1978 Jul;235(1):E53–E62. doi: 10.1152/ajpendo.1978.235.1.E53. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A., Vega F. V., Pointer R. H. Actions of insulin in fat cells. Effects of low temperature, uncouplers of oxidative phosphorylation, and respiratory inhibitors. J Biol Chem. 1977 Apr 10;252(7):2226–2233. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Studies of insulin insensitivity in soleus muscles of obese mice. Metabolism. 1978 Dec;27(12 Suppl 2):1982–1993. doi: 10.1016/s0026-0495(78)80014-6. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Gurny P. A., Lockwood D. H. Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J Biol Chem. 1977 Jan 25;252(2):560–562. [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin induced changes in insulin binding and insulin-sensitivity of adipocytes. Metabolism. 1978 Dec;27(12 Suppl 2):2009–2014. doi: 10.1016/s0026-0495(78)80017-1. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin-dependent regulation of the insulin-sensitivity of adipocytes. Nature. 1978 Jun 1;273(5661):394–396. doi: 10.1038/273394a0. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem. 1979 Oct 25;254(20):10153–10160. [PubMed] [Google Scholar]
- Martin M. S., Pohl S. L. Insulin-induced insulin resistance of alpha-aminoisobutyric acid transport in cultured human skin fibroblasts. J Biol Chem. 1979 Oct 25;254(20):9976–9978. [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977 Apr;100(4):1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes. 1977 Jul;26(7):680–688. doi: 10.2337/diab.26.7.680. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Siegel J., Olefsky J. M. Role of intracellular energy in insulin's ability to activate 3-O-methylglucose transport by rat adipocytes. Biochemistry. 1980 May 13;19(10):2183–2190. doi: 10.1021/bi00551a029. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Kahn C. R., Neville D. M., Jr Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J Biol Chem. 1975 Jun 25;250(12):4702–4707. [PubMed] [Google Scholar]
- Tsuruhara T., Dufau M. L., Cigorraga S., Catt K. J. Hormonal regulation of testicular luteinizing hormone receptors. Effects on cyclic AMP and testosterone responses in isolated Leydig cells. J Biol Chem. 1977 Dec 25;252(24):9002–9009. [PubMed] [Google Scholar]


