Abstract
Do cancer patients responding to immunotherapy have immunological profiles that influence the therapeutic outcome, or do they develop efficient antitumor responses only upon immunotherapy? We came across this “chicken or the egg” dilemma when treating secondary liver tumors with Semliki Forest viruses expressing interleukin-12. In our system, the “egg,” that is, the pre-treatment immunological profile, seemed to make the difference. The properties of an effective antitumor response were also defined.
Keywords: IL-12, IL-15Rα, alphavirus, cancer immunotherapy, liver secondary tumor
Cancer has become one of the principal causes of death in developed countries. Liver neoplasms are very aggressive and current therapeutic options for primary or secondary tumors affecting the liver include (but are not limited to) surgery, chemotherapy and radiotherapy. Despite considerable advances in the detection and treatment of liver cancers, a high percentage of patients still does not respond to therapy. Recently, immunotherapy has emerged as a promising approach against various types of cancer, either as a standalone intervention or combined with the aforementioned regimens. Immunotherapy relies on the capacity of the immune system to recognize and destroy cancer cells. However, malignant cells, jointly with stromal cells, can modulate the immune system and promote the development of an immunosuppressive milieu that counteracts immune responses.1 In this sense, the administration of immunomodulating monoclonal antibodies2 or cytokines (e.g., interleukin-12, IL-12)3 that are able to overcome immunosuppression or stimulate antitumor responses, has opened a new therapeutic avenues. Nevertheless, in many cases the responses of cancer patients to immunotherapy are limited, mostly due to the strong immunosuppressive microenvironment established within tumors and/or to a weak activation of immune effector cells, resulting in therapeutic failure. We reasoned that by performing an in-depth analysis of the immune responses that are elicited in the context of anticancer immunotherapy, we could design more potent immunotherapeutic regimens. To this aim, we focused the immunological differences between animals that responded and those that did not respond to anticancer immunotherapy. The fact that we observed significant differences in these two groups raised a “chicken or the egg” dilemma: Did responding animals have a different immunological profile before treatment that helped them to respond better, or did they develop a different immunological profile as a result of immunotherapy?
Our experimental approach relied on the administration of a Semliki Forest virus-based vector expressing IL-12 (SFV-IL-12)4 into MC38 colon adenocarcinomas implanted in the liver of immunocompetent mice, a model commonly employed as a surrogate for secondary liver neoplasms. We had previously shown that SFV-IL-12 is very efficient in eliciting antitumor responses in different cancer models.5 Several reasons may account for its high efficacy, including (1) high expression levels of IL-12 within the tumor, (2) the death of infected cancer cells, allowing for the release of tumor-associated antigens that be taken up and cross-presented by antigen-presenting cells,6 and (3) the induction of type I interferon (IFN) responses through the activation of pattern recognition receptors, which robustly sustain the activation of the immune system.7 Of note, the transient nature of this vector limits the toxicity associated with the expression of IL-12.
We first observed that orthotopic liver tumors were significantly more resistant to SFV-IL-12 than the same tumors implanted subcutaneously (48% vs. 92% complete remissions).8 These data indicate that the tissue surrounding malignant cells may have a profound influence on the outcome of immunotherapy, a notion that should be taken into attentive consideration for the design of preclinical studies on cancer.
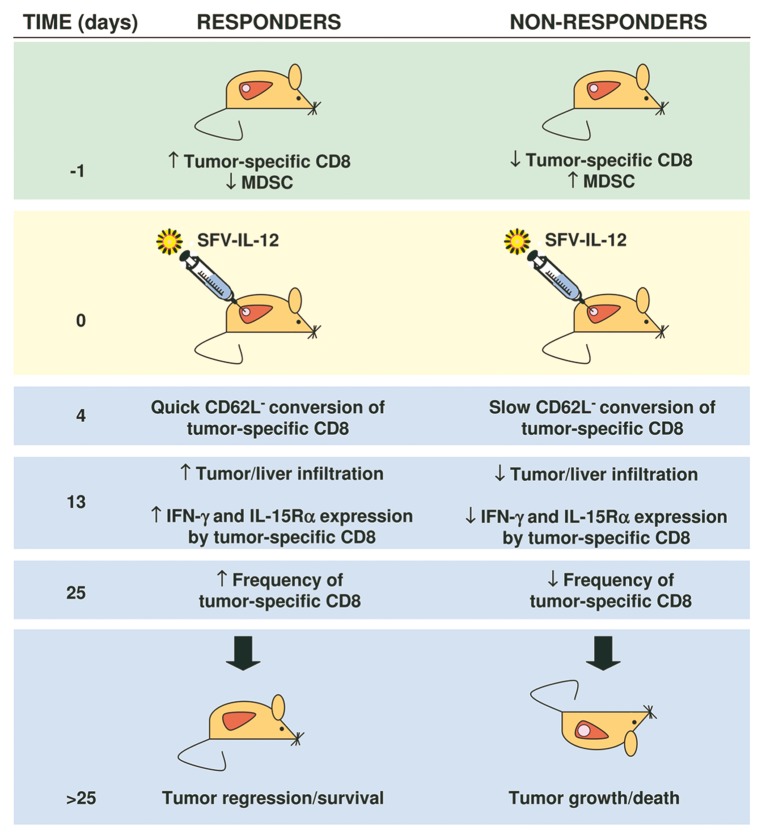
By comparing the animals that responded to SFV-IL-12 with those that did not, we were able to define important clues to determined therapeutic outcomes (Fig. 1).8 Interestingly, we observed that responders presented a higher number of tumor-specific CD8+ T-cells and a lower percentage of (Ly6C+ CD11b+) monocytic myeloid-derived suppressor cells (MDSCs) in the peripheral blood before treatment than non-responders, suggesting that animals with a high antitumor CD8+ T-cell/MDSC ratio at baseline might be prone to respond more efficiently to SFV-IL-12 therapy. Therefore, in this experimental setting the “egg” seems to come before the “chicken.” This is quite interesting because it implies that the success of immunotherapy can be predicted based on the immunological profile of patients at baseline, at least theoretically allowing for the pre-selection of patients with the highest probability to respond.
Figure 1. Immunological profiles of mice bearing secondary liver tumors before and after the administration of a Semliki Forest virus expressing interleukin-12. Responders included mice that completely rejected malignant cells or exhibited a reduction in tumor growth (partial efficacy). Non-responders included mice in which tumors grew at the same pace than in control mice (not shown). IFNγ, interferon γ.
Why did mice with a high antitumor CD8+ T-cell/MDSC ratio at baseline best respond to treatment? According to our study, after treatment, tumor-specific CD8+ T cells from responders acquired an effector-like phenotype, defined by the loss of CD62L, earlier than those of non-responders. This phenomenon may reflect the importance of establishing a potent effector antitumor response before tumor progression becomes unstoppable. On the other hand, since these changes were observed as early as four days after treatment, they might also be used to predict the outcome of therapy.
The speed at which antitumor immune responses are induced was not the only factor that determined the outcome of immunotherapy. We observed that tumor-specific CD8+ T cells from responding mice exhibited higher avidity for tumor-associated antigens and were more prone to infiltrate neoplastic lesions than those of non-responders. In addition, responders were able to maintain high levels of tumor-specific CD8+ T cells with enhanced effector functions for prolonged periods. Interestingly, tumor-infiltrating CD8+ T cells from mice responding to therapy overexpressed the IL-15 receptor α subunit (IL-15Rα), which may account, at least in part, for the sustained immune response observed in these animals.9
Overall, our findings suggest that the induction of an early and robust CD8+ T-cell response that persist for a long time is required for the antineoplastic effects of SFV-IL-12. A possible way to promote sustained CD8+ T-cell responses is by providing IL-15. In our particular case, this cytokine seems to be an optimal choice, since the overexpression of its receptor by CD8+ T-cells was associated with better therapeutic responses. In fact, preliminary results obtained by our group indicate that IL-15 is able to potentiate the antitumor potential of SFV-IL-12, increasing the proportion of animals that completely reject neoplastic lesions and survive.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24499
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Madoz JR, Prieto J, Smerdou C. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol Ther. 2005;12:153–63. doi: 10.1016/j.ymthe.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Quetglas JI, Ruiz-Guillen M, Aranda A, Casales E, Bezunartea J, Smerdou C. Alphavirus vectors for cancer therapy. Virus Res. 2010;153:179–96. doi: 10.1016/j.virusres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5:823–7. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidmark AS, Nordström EK, Dosenovic P, Forsell MN, Liljeström P, Karlsson Hedestam GB. Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling. J Virol. 2006;80:7100–10. doi: 10.1128/JVI.02579-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quetglas JI, Rodriguez-Madoz JR, Bezunartea J, Ruiz-Guillen M, Casales E, Medina-Echeverz J, et al. Eradication of liver-implanted tumors by semliki forest virus expressing IL-12 requires efficient long-term immune responses. J Immunol. 2013;190:2994–3004. doi: 10.4049/jimmunol.1201791. [DOI] [PubMed] [Google Scholar]
- 9.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]