Abstract
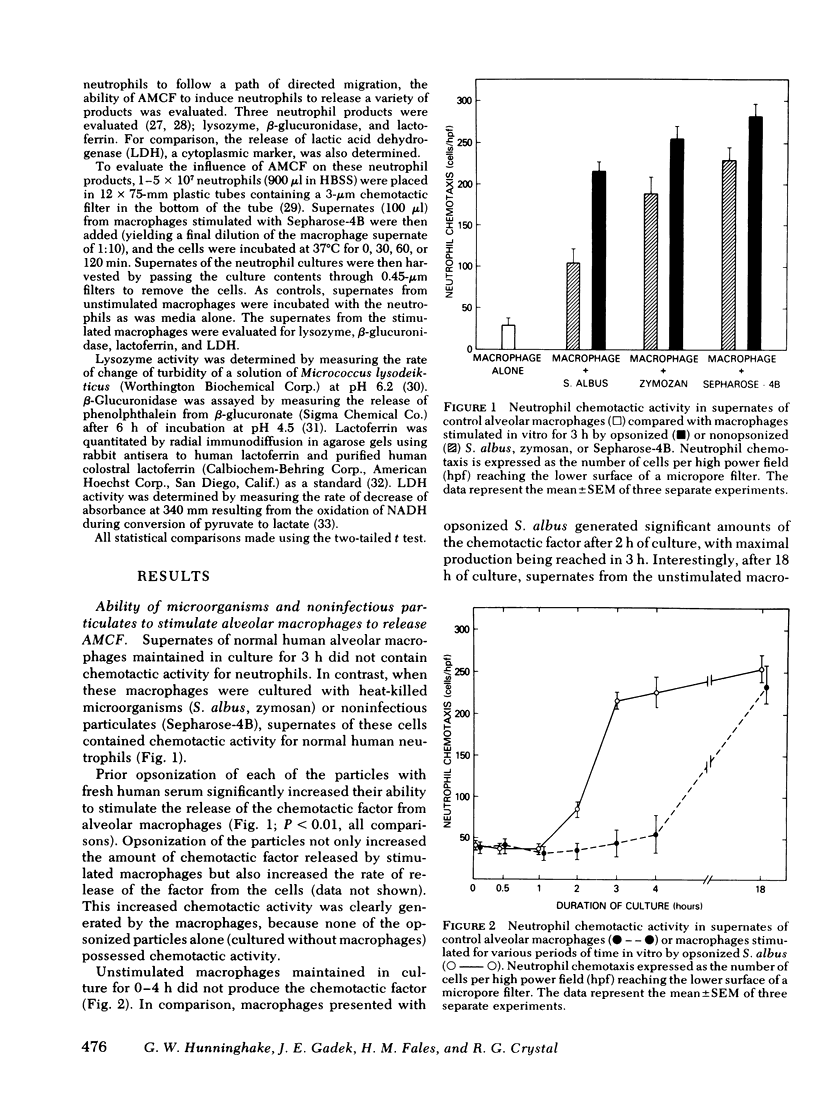
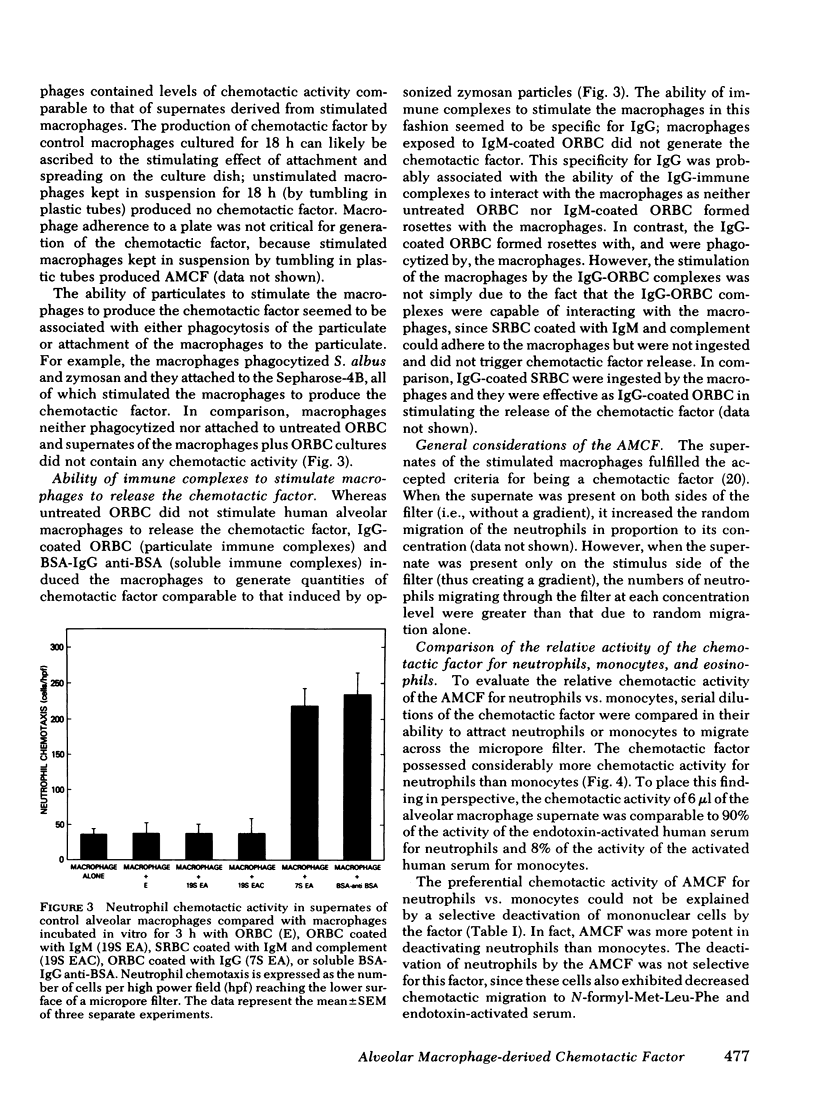
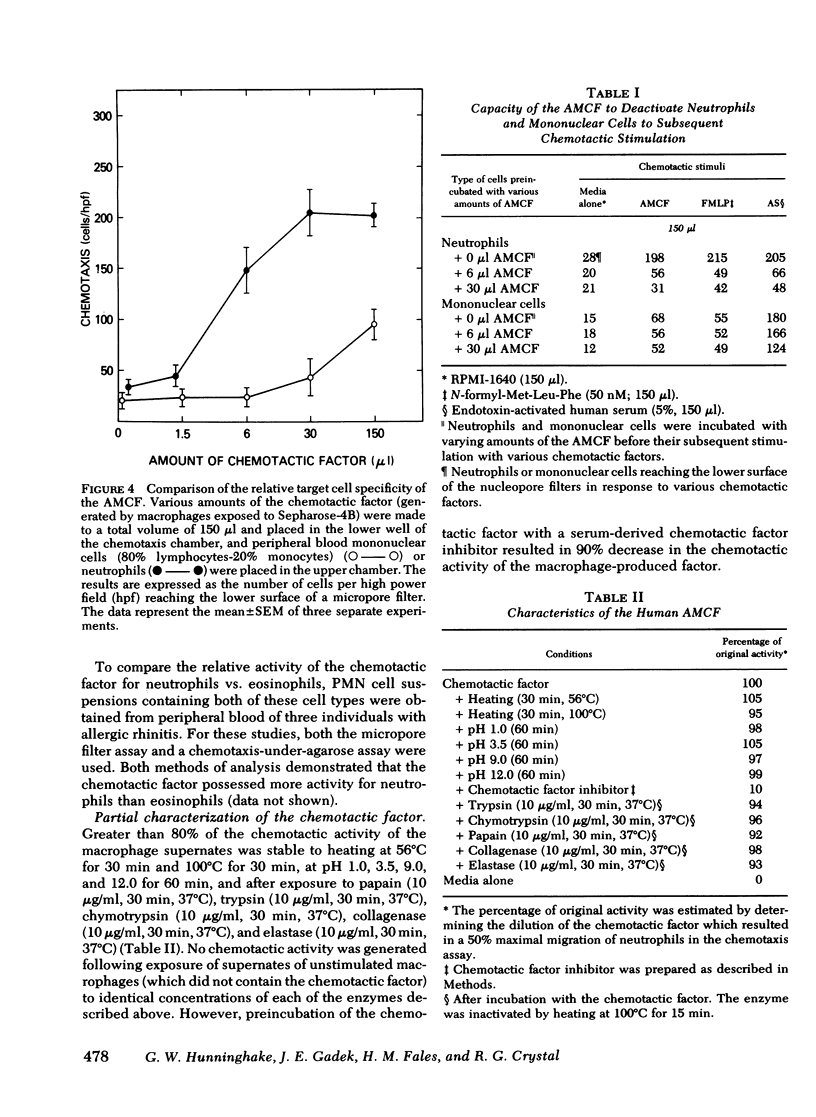
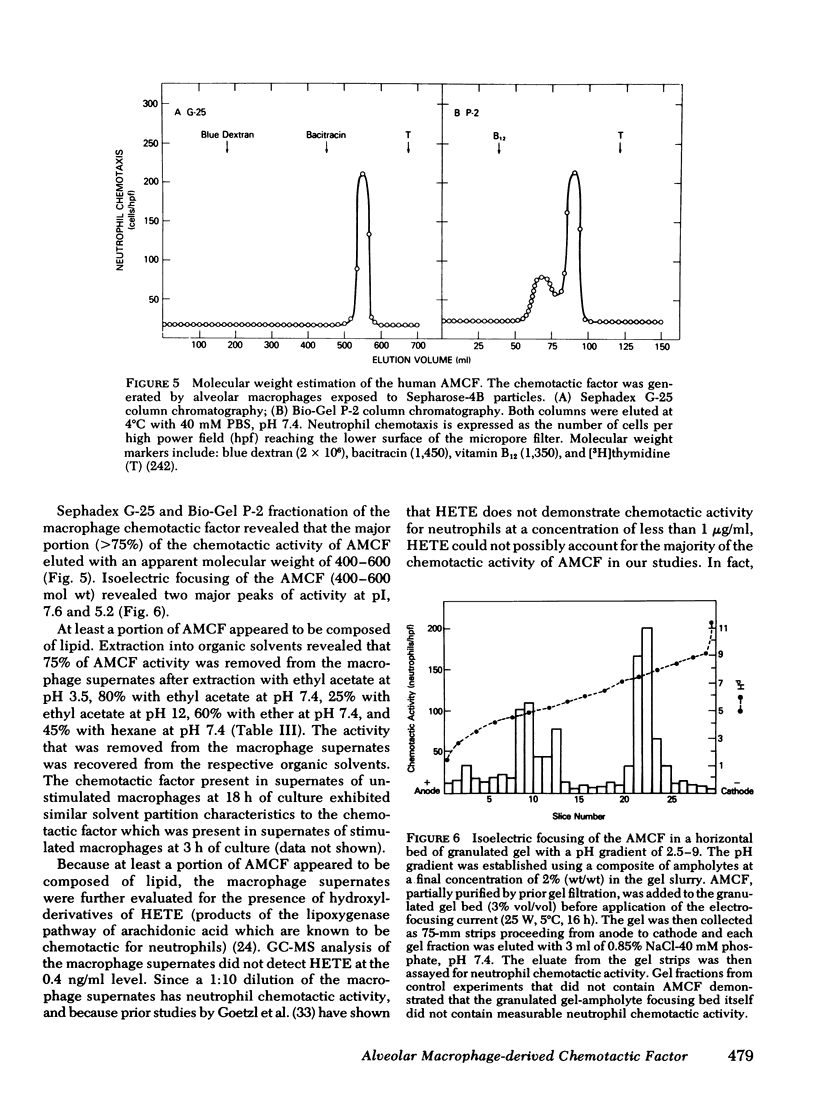
The presence of neutrophils within the lung is a characteristic feature of a variety of lung diseases. To evaluate the potential role of alveolar macrophages in modulating the migration of neutrophils to the lung, normal human alveolar macrophages obtained from volunteers by bronchopulmonary lavage, were exposed for various periods of time in vitro to heat-killed microorganisms, and noninfectious particulates, immune complexes, and the macrophage supernates were evaluated for chemotactic activity. The microorganisms, noninfectious particulates, and immune complexes were chosen as stimuli for alveolar macrophages because these stimuli are representative of a spectrum of pathogenic agents that cause neutrophil accumulation in the lower respiratory tract. After incubation with each of these stimuli, alveolar macrophages released low molecular weight (400-600) chemotactic factor(s) (alveolar macrophage-derived chemotactic factor[s] [AMCF]) with relatively more activity for neutrophils than monocytes or eosinophils. Checker-board analysis of the AMCF revealed that the factor was primarily chemotactic and not chemokinetic for neutrophils. The selectivity for neutrophils vs. monocytes could not be explained by a selective deactivation of monocytes, because the AMCF was more potent in deactivating neutrophils than monocytes. Partial characterization of AMCF demonstrated it was heterogeneous with the following features: (a) stable to heating at 56 and 100°C for 30 min; (b) stable over a pH range of 1.0 to 12.0 for 60 min; (c) stable after exposure to trypsin, papain, chymotrypsin, collagenase, and elastase; (d) partially inhibited by serum chemotactic factor inhibitor(s); (e) two major isoelectric points (pI 7.6 and 5.2); and (f) partially extractable into ethyl acetate, ether, and hexane. Although AMCF was, at least, partially lipid in nature, it did not appear to be similar to previously described lipid chemotactic factors (e.g., hydroxy-derivatives of 5,8,10,14-eicosatetraenoic acid); analysis by gas chromatography-mass spectrophotometry of AMCF extracted into ethyl acetate did not reveal the presence of 5,8,10,14-eicosatetraenoic acid. The macrophage supernates containing the AMCF also stimulated normal human neutrophils to release lysozyme and lactoferrin but not lactate dehydrogenase. These studies suggest that a wide variety of potentially pathogenic stimuli induce normal alveolar macrophages to generate a low molecular weight chemotactic factor(s) that preferentially attracts neutrophils. Because alveolar macrophages are normal residents of alveoli, it is likely that by releasing this factor(s) macrophages play a significant role in amplifying the inflammatory processes seen in many acute and chronic lung diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med. 1978 Feb 16;298(7):353–357. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kirkpatrick C. H. Chemotactic activity in dialyzable transfer factor. Proc Natl Acad Sci U S A. 1974 Feb;71(2):498–502. doi: 10.1073/pnas.71.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Tashjian A. H., Jr, Rubin R. H., Austen K. F. Production of a low molecular weight eosinophil polymorphonuclear leukocyte chemotactic factor by anaplastic squamous cell carcinomas of human lung. J Clin Invest. 1978 Mar;61(3):770–780. doi: 10.1172/JCI108991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. The J. Burns Amberson Lecture--in defense of the lung. Am Rev Respir Dis. 1970 Nov;102(5):691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Goetzl E. J., Austen K. F. The fibrinolytic pathway of human plasma. II. The generation of chemotactic activity by activation of plasminogen proactivator. J Clin Invest. 1973 Oct;52(10):2591–2595. doi: 10.1172/JCI107451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Kay A. B., Austen K. F. A prealbumin activator of prekallikrein. 3. Appearance of chemotactic activity for human neutrophils by the conversion of human prekallikrein to kallikrein. J Exp Med. 1972 Jan;135(1):81–97. doi: 10.1084/jem.135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Stechschulte D. J., Austen K. F. An eosinophil leukocyte chemotactic factor of anaphylaxis. J Exp Med. 1971 Mar 1;133(3):602–619. doi: 10.1084/jem.133.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Fiegel V. D., Simmons R. L. Chemotaxis of human polymorphonuclear neutrophils under agarose: morphologic changes associated with the chemotactic response. J Immunol. 1976 Nov;117(5 Pt 1):1676–1683. [PubMed] [Google Scholar]
- REEVES W. J., Jr, FIMOGNARI G. M. AN IMPROVED PROCEDURE FOR THE PREPARATION OF CRYSTALLINE LACTIC DEHYDROGENASE FROM HOG HEART. J Biol Chem. 1963 Dec;238:3853–3858. [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;Series 47:72–98. [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Weinberger S. E., Kelman J. A., Elson N. A., Young R. C., Jr, Reynolds H. Y., Fulmer J. D., Crystal R. G. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978 Oct;89(4):459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Malawista S. E. The mobilization and extracellular release of granular enzymes from human leukocytes during phagocytosis. J Cell Biol. 1972 Jun;53(3):788–797. doi: 10.1083/jcb.53.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M., Mayumi M., Yamamoto S., Hayashi H. Immunoglobulin G as possible precursor of chemotactic factor. Nature. 1970 Mar 21;225(5238):1138–1139. doi: 10.1038/2251138a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


