Abstract
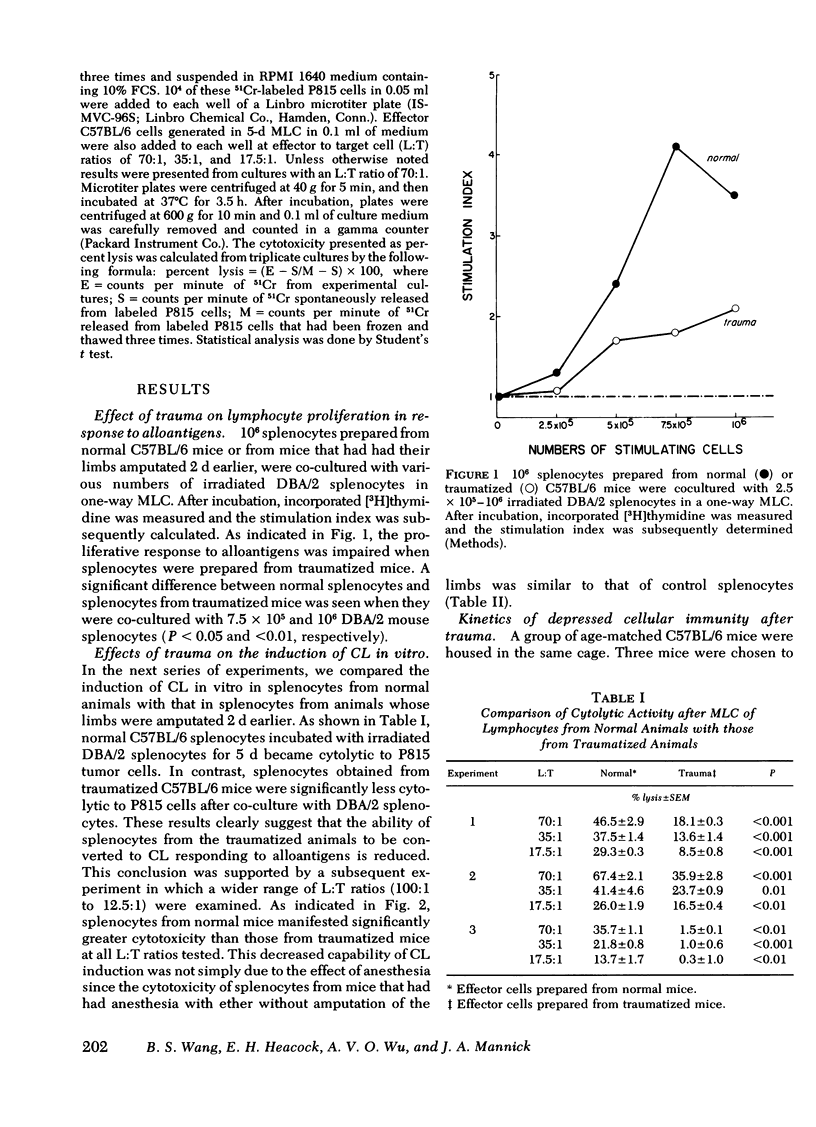
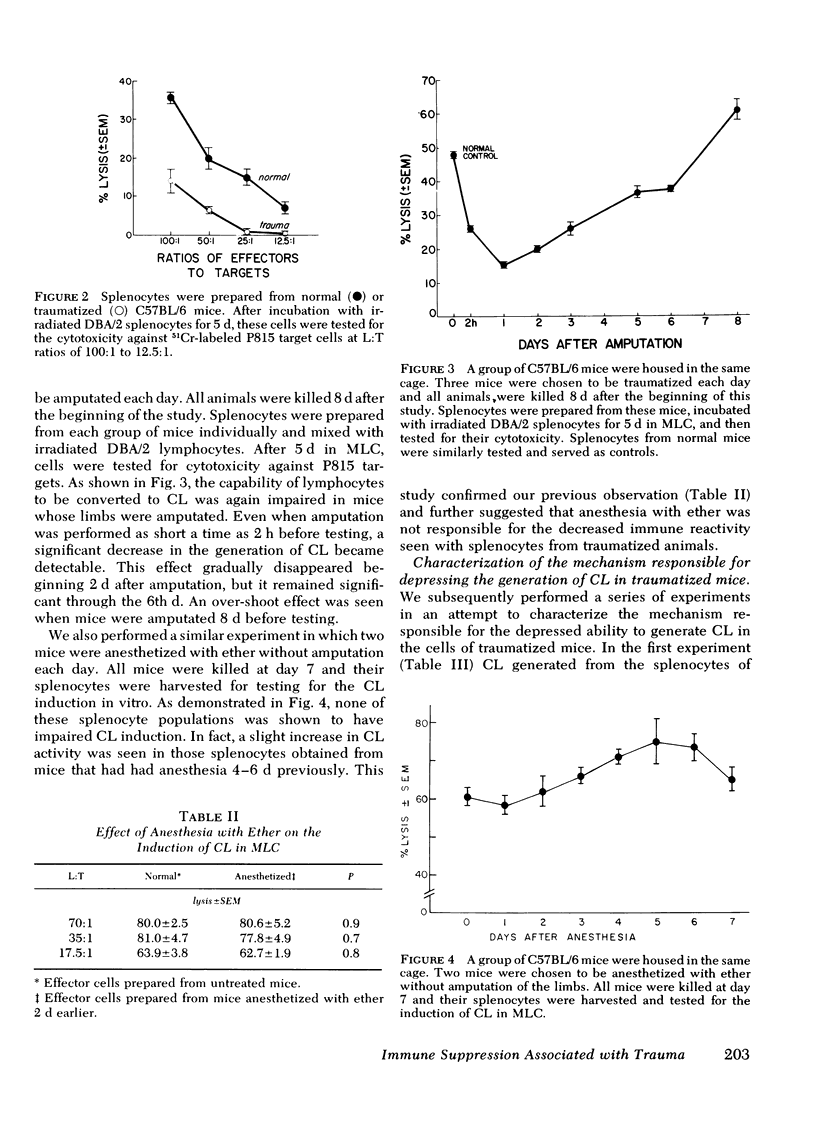
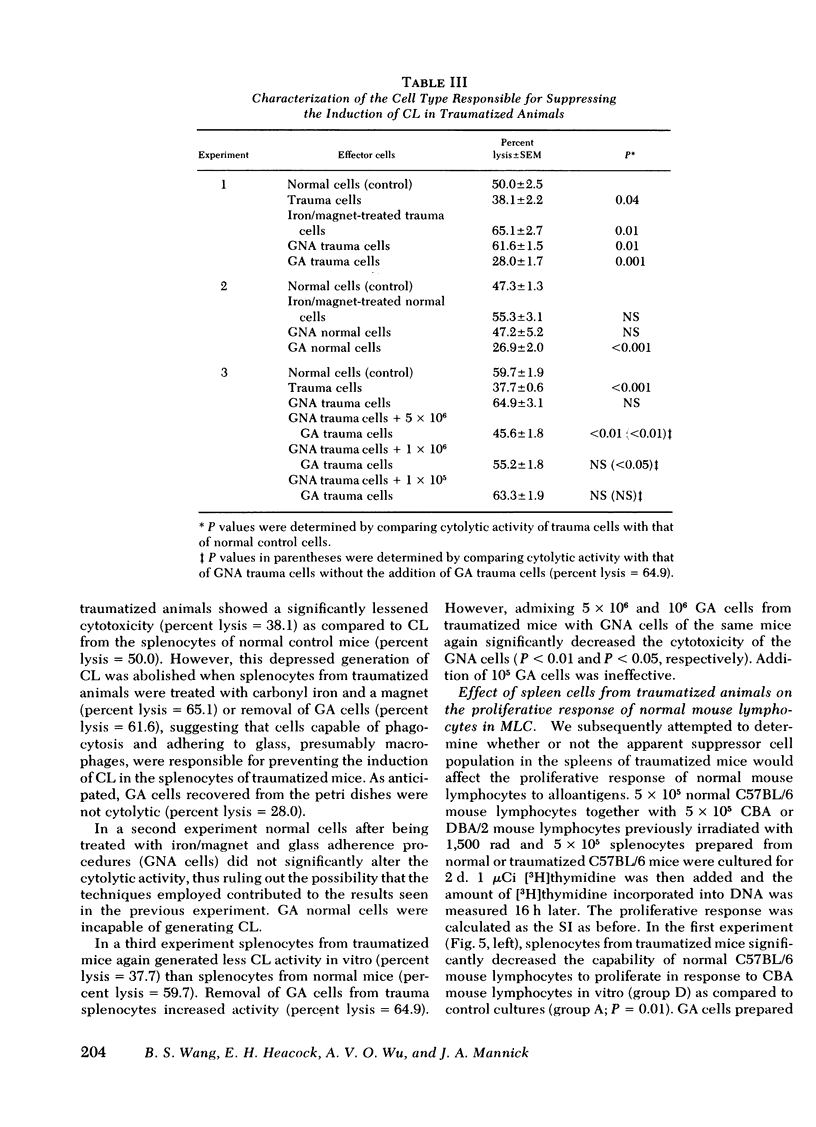
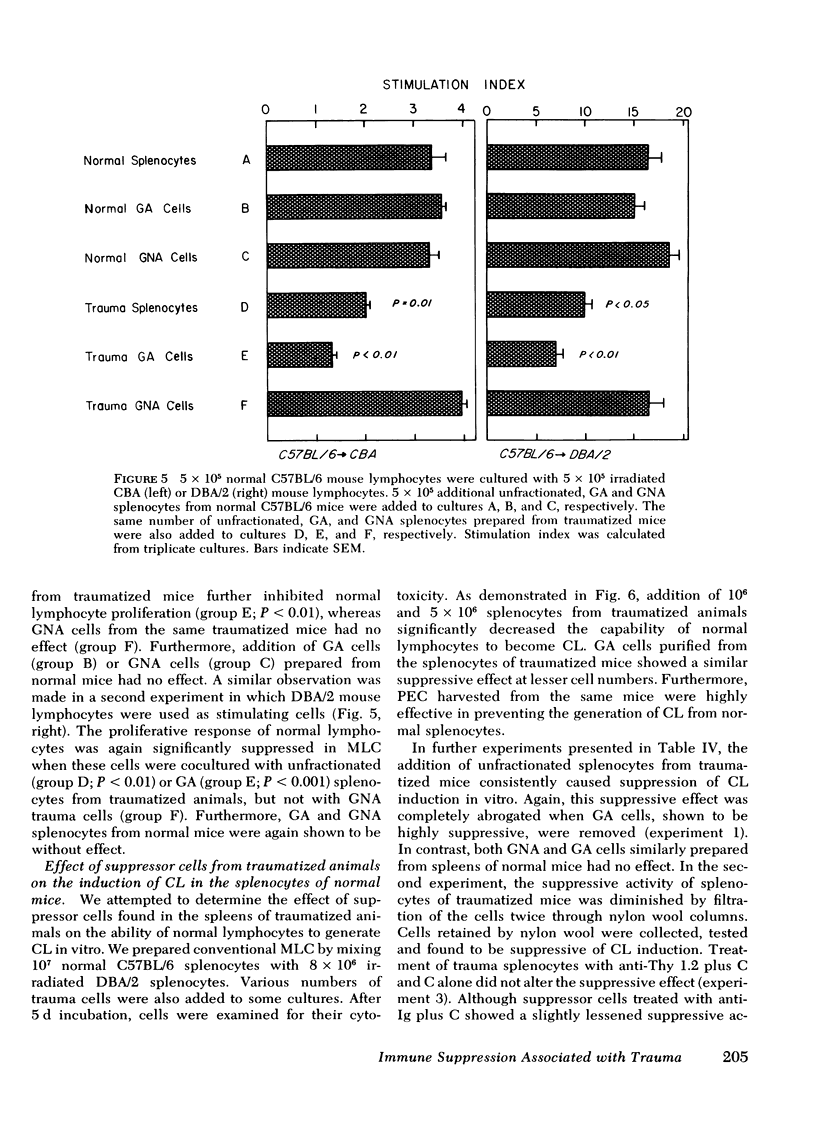
Immunoincompetency is often seen in patients after various types of trauma and is associated with increased morbidity and mortality from infectious complications. To understand better the immunologic impairment associated with trauma, we have studied this phenomenon in an animal model. Splenocytes from mice traumatized by amputation of their right hind limbs were consistently shown to have a diminished capacity to proliferate in response to alloantigens and to form alloreactive cytolytic cells in mixed lymphocyte cultures. Anesthesia itself had no effect in this system. The immunoincompetency was detected from 2 h to 6 d after surgical trauma and was completely reversed by removing adherent and phagocytic cells from the splenocytes. Furthermore, addition of splenocytes from traumatized mice to mixed lymphocyte cultures from normal mice prevented normal lymphocytes from responding to alloantigens, suggesting the existence of suppressor cells. The suppressor cells were found to adhere to glass and to nylon wool columns, and were contained within an esterase-positive cell population. They were insensitive to treatment with anti-Thy 1.2 and anti-Ig sera in the presence of complement. Therefore, the present results suggest that a Thy 1.2-negative, Ig-negative, esterase-positive cell population capable of adhering to glass and nylon wool, presumably macrophages, was responsible for the inhibition of the responsiveness of lymphocytes to alloantigens in traumatized animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Hegg M., Altemeier W. A. Neutrophil function in selected surgical disorders. Ann Surg. 1968 Sep;168(3):447–458. doi: 10.1097/00000658-196809000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Moncrief J. A. Alterations of the immune response following severe thermal injury. Arch Surg. 1966 Jul;93(1):75–83. doi: 10.1001/archsurg.1966.01330010077011. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., Stinnett J. D., Ogle C. K., Ogle J. D., Morris M. J. A comparison of immunologic profiles and their influence on bacteremia in surgical patients with a high risk of infection. Surgery. 1979 Jul;86(1):94–104. [PubMed] [Google Scholar]
- Chambler K., Batchelor J. R. Influence of defined incompatibilities and area of burn on skin-homograft survival in burned-subjects. Lancet. 1969 Jan 4;1(7584):16–18. doi: 10.1016/s0140-6736(69)90984-2. [DOI] [PubMed] [Google Scholar]
- Christou N. V., Meakins J. L. Neutrophil function in anergic surgical patients: neutrophil adherence and chemotaxis. Ann Surg. 1979 Nov;190(5):557–564. doi: 10.1097/00000658-197911000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantian M. B. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978 Aug;188(2):209–215. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantian M. B., Menzoian J. O., Nimberg R. B., Schmid K., Mannick J. A. Association of a circulating immunosuppressive polypeptide with operative and accidental trauma. Ann Surg. 1977 Jan;185(1):73–79. doi: 10.1097/00000658-197701000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. J., Irvine J. M., Turnbull A. R. Depression of immunological responses due to surgery. A model in the guinea-pig. Immunology. 1974 Sep;27(3):393–399. [PMC free article] [PubMed] [Google Scholar]
- Elgert K. D., Farrar W. L. Suppressor cell activity in tumor-bearing mice. I. Dualistic inhibition by suppressor T lymphocytes and macrophages. J Immunol. 1978 Apr;120(4):1345–1353. [PubMed] [Google Scholar]
- Fernbach B. R., Kirchner H., Herberman R. B. Inhibition of the mixed lymphocyte culture by peritoneal exudate cells. Cell Immunol. 1976 Mar 15;22(2):399–403. doi: 10.1016/0008-8749(76)90043-5. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kinnaert P., Mahieu A., Van Geertruyden N. Stimulation of antibody synthesis induced by surgical trauma in rats. Clin Exp Immunol. 1978 May;32(2):243–252. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Muchmore A. V., Chused T. M., Holden H. T., Herberman R. B. Inhibition of proliferation of lymphoma cells and T lymphocytes by suppressor cells from spleens of tumor-bearing mice. J Immunol. 1975 Jan;114(1 Pt 1):206–210. [PubMed] [Google Scholar]
- LEWIS M. R., COLE W. H. Experimental increase of lung metastases after operative trauma (amputation of limb with tumor). AMA Arch Surg. 1958 Oct;77(4):621–626. doi: 10.1001/archsurg.1958.04370010153015. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Crosby W. H. Histochemical characterization of cellular and structural elements of the human spleen. J Histochem Cytochem. 1972 Dec;20(12):1049–1058. doi: 10.1177/20.12.1049. [DOI] [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. EFFECT OF BURNS IN RATS ON DEFENSE MECHANISMS AGAINST PSEUDOMONAS AERUGINOSA. J Infect Dis. 1965 Apr;115:159–170. doi: 10.1093/infdis/115.2.159. [DOI] [PubMed] [Google Scholar]
- Mahler D., Batchelor J. R. Phytohaemagglutinin transformation of lymphocytes in burned patients. Transplantation. 1971 Nov;12(5):409–411. doi: 10.1097/00007890-197111000-00015. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E. T., LaJohn L. A. The effect of thermal trauma in mice on cytotoxicity of lymphocytes. Proc Soc Exp Biol Med. 1977 Jan;154(1):72–77. doi: 10.3181/00379727-154-39607. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E., Evans G. Antibody production in mice after thermal and tourniquet trauma. Surgery. 1967 Jun;61(6):896–903. [PubMed] [Google Scholar]
- McLoughlin G. A., Wu A. V., Saporoschetz I., Nimberg R., Mannick J. A. Correlation between anergy and a circulating immunosuppressive factor following major surgical trauma. Ann Surg. 1979 Sep;190(3):297–304. doi: 10.1097/00000658-197909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakins J. L., Pietsch J. B., Bubenick O., Kelly R., Rode H., Gordon J., MacLean L. D. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977 Sep;186(3):241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M., Eurenius K., Katz R. M., Canales L., Foley F. D., Mortensen R. F. Cell-mediated immunity after thermal injury. Ann Surg. 1973 Feb;177(2):139–143. doi: 10.1097/00000658-197302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M., Eurenius K., Mortensen R. F., Mason A. D., Jr Ability of splenic lymphocytes from injured rats to induce a graft-versus-host reaction. Transplantation. 1972 Jul;14(1):106–108. doi: 10.1097/00007890-197207000-00015. [DOI] [PubMed] [Google Scholar]
- Munster A. M. Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet. 1976 Jun 19;1(7973):1329–1330. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- Neilan B. A., Taddeini L., Strate R. G. T lymphocyte rosette formation after major burns. JAMA. 1977 Aug 8;238(6):493–496. [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Wachtel T. L. Thermal injury-associated immunosuppression: occurrence and in vitro blocking effect of post recovery serum. J Immunol. 1979 May;122(5):1736–1741. [PubMed] [Google Scholar]
- Ottesen E. A. Modulation of the host response in human schistosomiasis. I. Adherent suppressor cells that inhibit lymphocyte proliferative responses to parasite antigens. J Immunol. 1979 Oct;123(4):1639–1644. [PubMed] [Google Scholar]
- PERKINS E. H., MAKINODAN T. THE SUPPRESSIVE ROLE OF MOUSE PERITONEAL PHAGOCYTES IN AGGLUTININ RESPONSE. J Immunol. 1965 May;94:765–777. [PubMed] [Google Scholar]
- Park S. K., Brody J. I., Wallace H. A., Blakemore W. S. Immunosuppressive effect of surgery. Lancet. 1971 Jan 9;1(7689):53–55. doi: 10.1016/s0140-6736(71)90777-x. [DOI] [PubMed] [Google Scholar]
- Parker D. J., Cantrell J. W., Karp R. B., Stroud R. M., Digerness S. B. Changes in serum complement and immunoglobulins following cardiopulmonary bypass. Surgery. 1972 Jun;71(6):824–827. [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Pietsch J. B., Meakins J. L., MacLean L. D. The delayed hypersensitivity response: application in clinical surgery. Surgery. 1977 Sep;82(3):349–355. [PubMed] [Google Scholar]
- Quan P. C., Burtin P. Demonstration of nonspecific suppressor cells in the peripheral lymphocytes of cancer patients. Cancer Res. 1978 Feb;38(2):288–296. [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J. Kinetics of humoral responsiveness in severe thermal injury. Ann Surg. 1976 Jul;184(1):51–59. doi: 10.1097/00000658-197607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport F. T., Milgrom F., Kano K., Gesner B., Solowey A. C., Casson P., Silverman H. I., Converse J. M. Immunologic sequelae of thermal injury. Ann N Y Acad Sci. 1968 Aug 14;150(3):1004–1008. doi: 10.1111/j.1749-6632.1968.tb14753.x. [DOI] [PubMed] [Google Scholar]
- Ritzmann S. E., Larson D. L., McClung C., Abston S., Falls D., Goldman A. S. Immunoglobulin levels in burned patients. Lancet. 1969 Jun 7;1(7606):1152–1153. doi: 10.1016/s0140-6736(69)91670-5. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Antikatzides T. G. Decreased resistance to intravenous tumour-cell challenge during reticuloendothelial depression following surgery. Br J Cancer. 1976 Oct;34(4):381–389. doi: 10.1038/bjc.1976.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Daniels J. C., Beathard G. A., Lewis S. R., Lynch J. B., Ritzmann S. E. Mixed lymphocyte culture reaction in patients with acute thermal burns. J Trauma. 1974 Jan;14(1):53–57. doi: 10.1097/00005373-197401000-00007. [DOI] [PubMed] [Google Scholar]
- Todd C. W., Goodgame R. W., Colley D. G. Immune responses during human schistosomiasis mansoni. V. Suppression of schistosome antigen-specific lymphocyte blastogenesis by adherent/phagocytic cells. J Immunol. 1979 Apr;122(4):1440–1446. [PubMed] [Google Scholar]
- Veit B. C., Feldman J. D. Altered lymphocyte functions in rats bearing syngeneic Moloney sarcoma tumors. II. Suppressor cells. J Immunol. 1976 Aug;117(2):655–660. [PubMed] [Google Scholar]
- Zembala M., Mytar B., Popiela T., Asherson G. L. Depressed in vitro peripheral blood lymphocyte response to mitogens in cancer patients: the role of suppressor cells. Int J Cancer. 1977 May 15;19(5):605–613. doi: 10.1002/ijc.2910190503. [DOI] [PubMed] [Google Scholar]


