Abstract
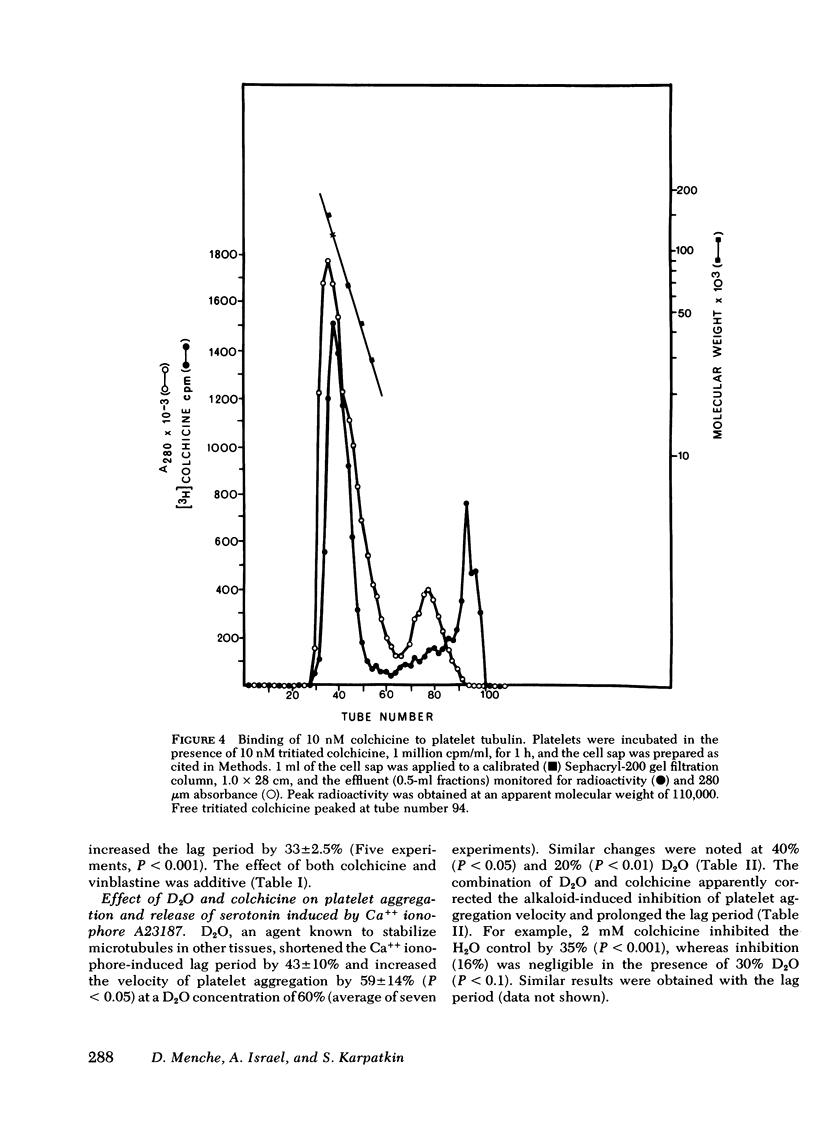
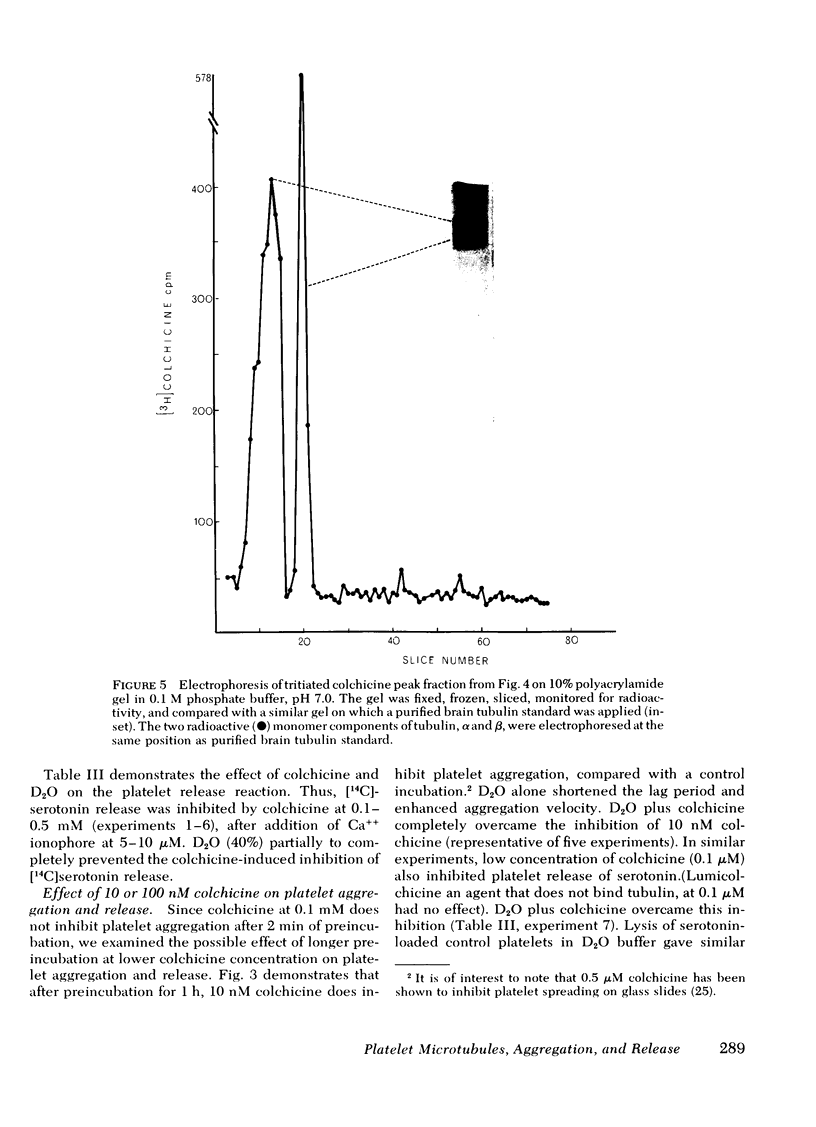
We examined the role of microtubules in platelet aggregation and secretion (release reaction) induced by the calcium ionophore A23187 (0.8-5 μM). At these concentrations, platelet aggregation was preceded by a lag period of ∼1 min. Colchicine (an agent that disrupts microtubule assembly-disassembly) was shown to bind to platelet microtubules by employing [3H]colchicine at a concentration that is specific for microtubules in other tissues (10 nM). Colchicine prolonged the lag period, inhibited the secondary wave of platelet aggregation, and inhibited the release reaction (release of [14C]serotonin). Platelets were next incubated with 20-60% D2O, an agent that stabilizes microtubules. D2O overcame colchicine-induced inhibition of the lag period, aggregation, and release. D2O alone enhanced platelet aggregation by 59±14% (SEM) and shortened the lag period by 43±10%. We conclude that functioning microtubules are required for platelet aggregation and release, and that microtubules of platelet preparations are functioning submaximally.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke O. Further studies on microtubules. A marginal bundle in human and rat thrombocytes. J Ultrastruct Res. 1965 Dec;13(5):469–477. doi: 10.1016/s0022-5320(65)90009-2. [DOI] [PubMed] [Google Scholar]
- Boyle Kay M. M., Fudenberg H. H. Inhibition and reversal of platelet activation by cytochalasin B or colcemid. Nature. 1973 Aug 3;244(5414):288–289. doi: 10.1038/244288a0. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechatelet L. R., Cooper M. R., McCall C. E. Dissociation by colchicine of the hexose monophosphate shunt activation from the bactericidal activity of the leukocyte. Infect Immun. 1971 Jan;3(1):66–72. doi: 10.1128/iai.3.1.66-72.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B., Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975 Nov;66(5):561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger S. E., Howell R. R., Seegmiller J. E. Suppression of metabolic accompaniments of phagocytosis by colchicine. Arthritis Rheum. 1965 Dec;8(6):1112–1122. doi: 10.1002/art.1780080610. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Effects of colchicine on collagenase in cultures of rheumatoid synovium. Arthritis Rheum. 1971 Nov-Dec;14(6):669–684. doi: 10.1002/art.1780140602. [DOI] [PubMed] [Google Scholar]
- Krishan A., Hsu D. Vinblastine-induced ribosomal complexes. Effect of some metabolic inhibitors on their formation and structure. J Cell Biol. 1971 Jun;49(3):927–932. doi: 10.1083/jcb.49.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Stanford N., Majerus P. W. Thrombin-induced protein phosphorylation in human platelets. J Clin Invest. 1975 Oct;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Brown D., Jeanrenaud B. Inhibitory effect of colchicine on amylase secretion by rat parotid glands. Possible localization in the Golgi area. J Cell Biol. 1977 Jun;73(3):578–593. doi: 10.1083/jcb.73.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon J. M. Effect of mitosis inhibitors on blood platelet microtubules and aggregation. J Physiol. 1971 Apr;214(1):145–158. doi: 10.1113/jphysiol.1971.sp009424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppitt G. D., Mitchell J. R. The effect of colchicine on human platelet behaviour. J Atheroscler Res. 1969 Sep-Oct;10(2):247–252. doi: 10.1016/s0368-1319(69)80012-8. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Edds K. T. Microtubules: structure, chemistry, and function. Physiol Rev. 1976 Oct;56(4):709–777. doi: 10.1152/physrev.1976.56.4.709. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Collier B., Lastowecka A., Stern D. Inhibition by colchicine and by vinblastine of acetylcholine-induced catecholamine release from the adrenal gland: an anticholinergic action, not an effect upon microtubules. Mol Pharmacol. 1972 Mar;8(2):264–267. [PubMed] [Google Scholar]
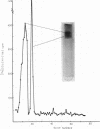
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White J. G. Effects of colchicine and Vinca alkaloids on human platelets. I. Influence on platelet microtubules and contractile function. Am J Pathol. 1968 Aug;53(2):281–291. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Effects of colchicine and vinca alkaloids on human platelets. 3. Influence on primary internal contraction and secondary aggregation. Am J Pathol. 1969 Mar;54(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]