Abstract
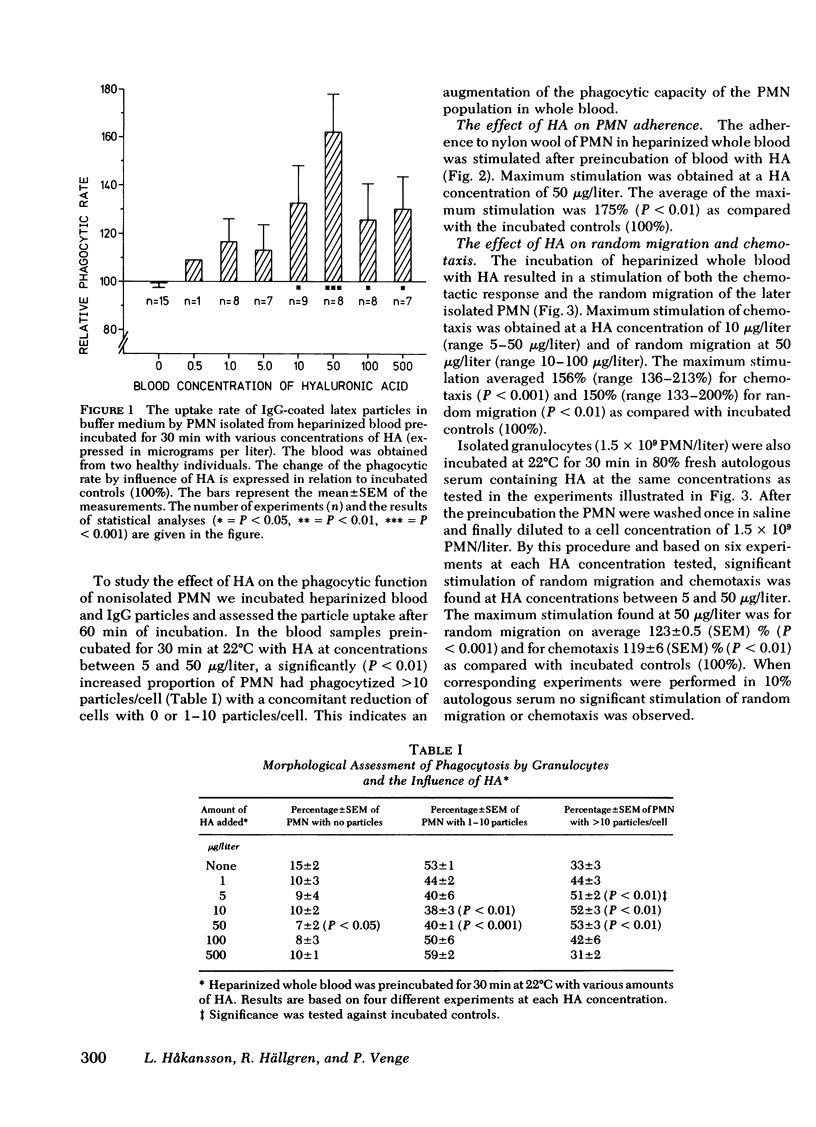
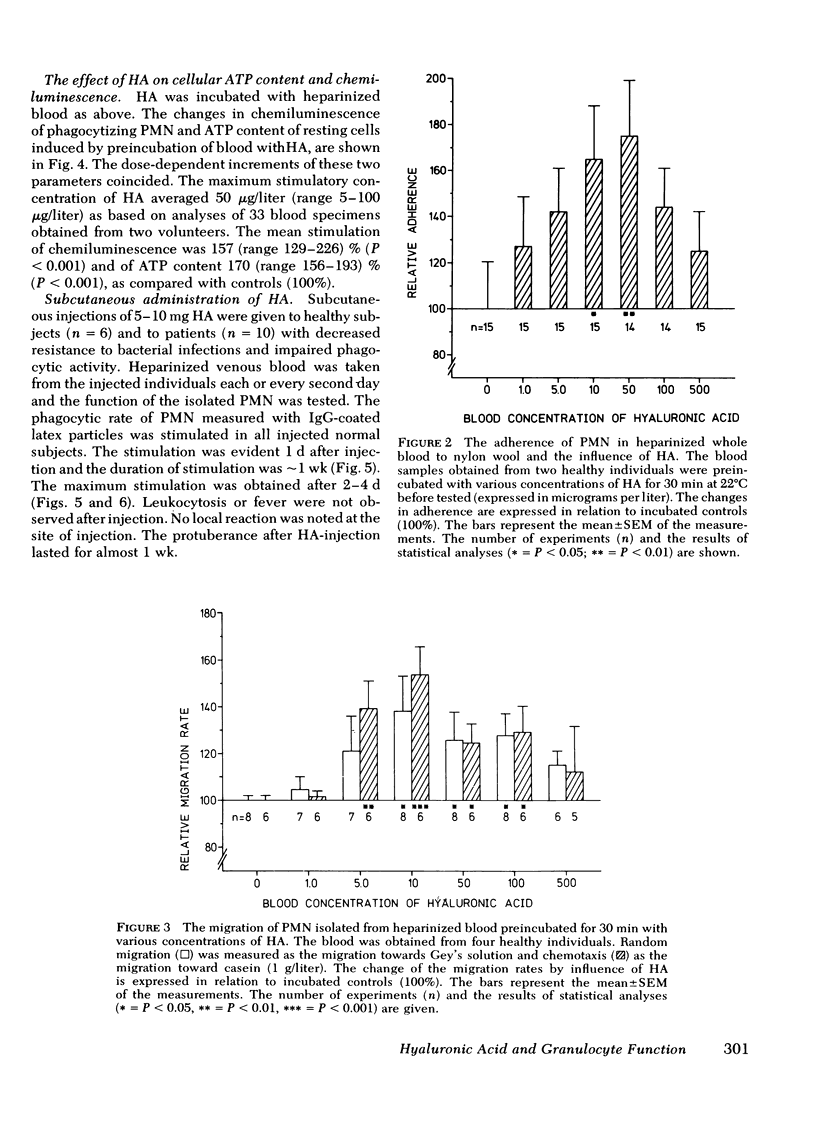
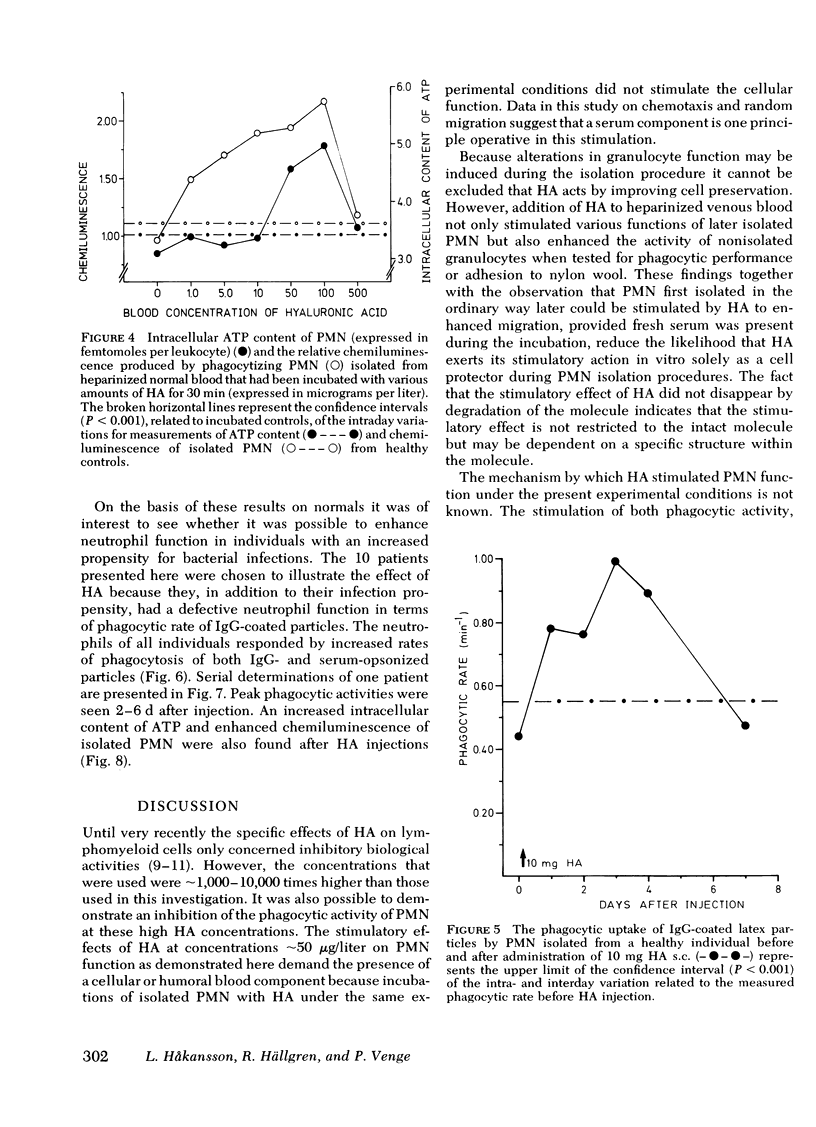
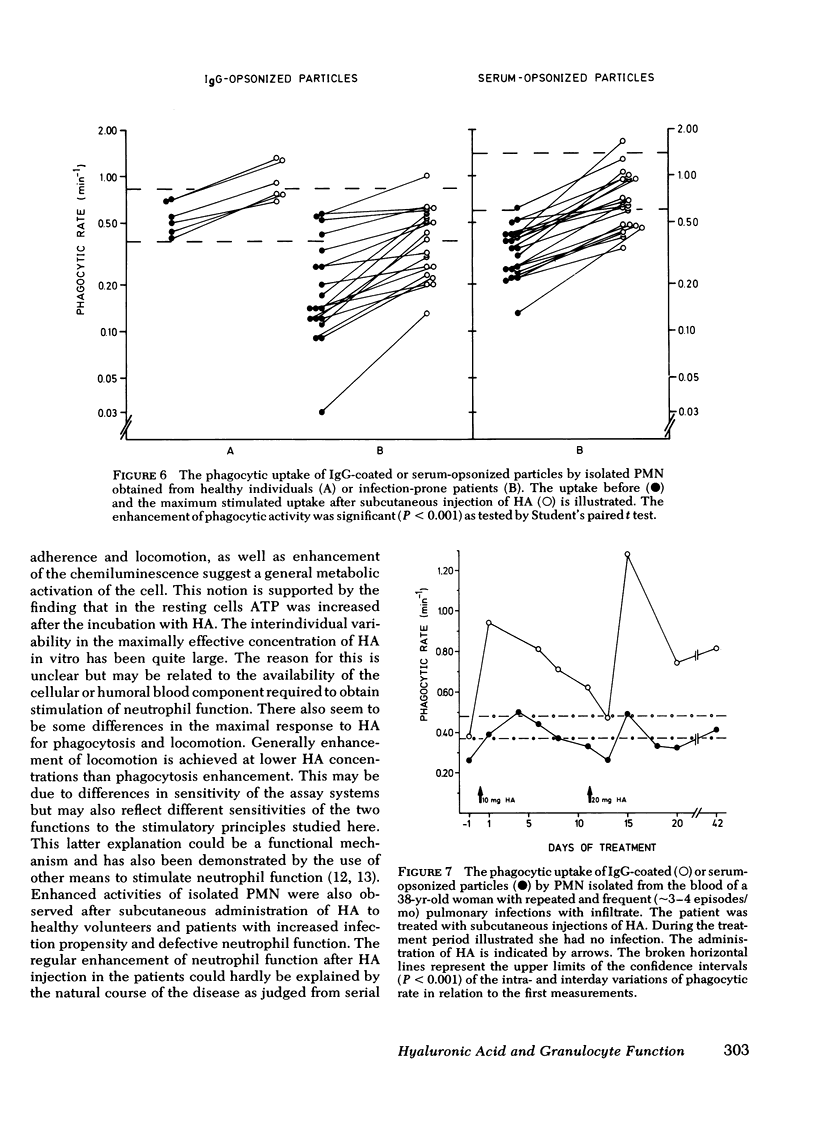
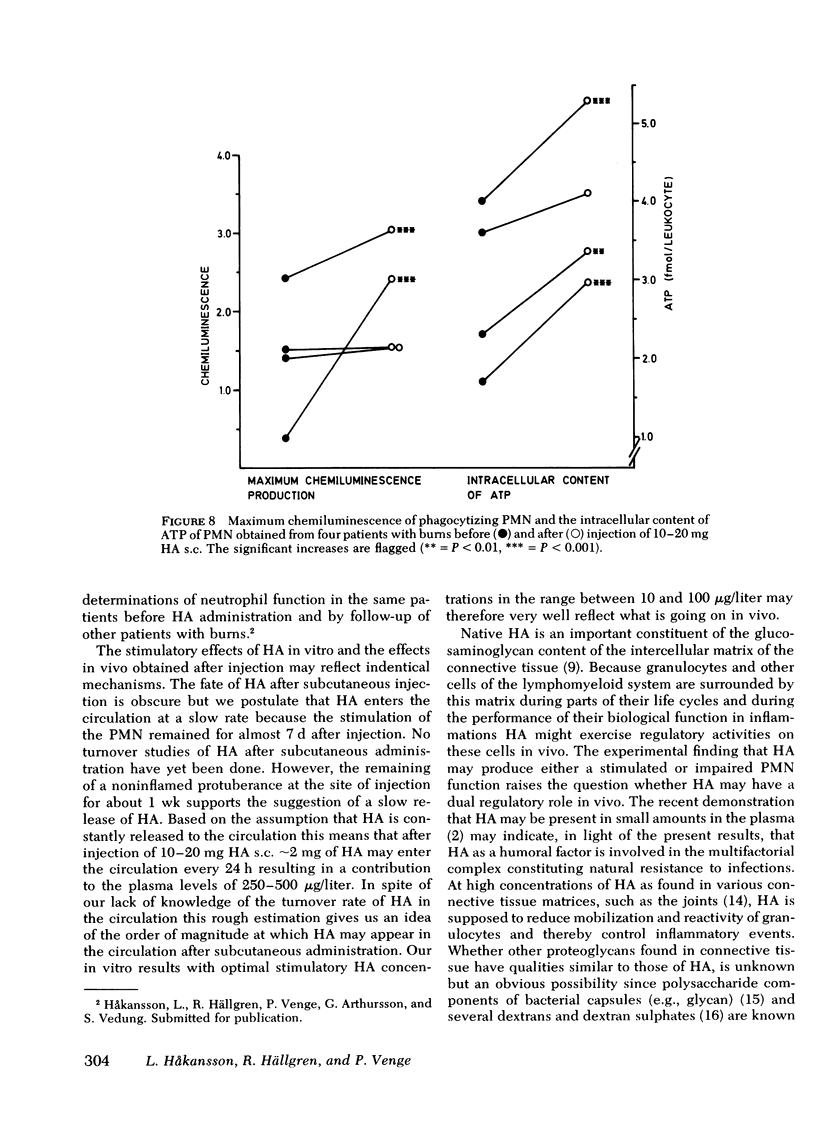
Hyaluronic acid (HA) stimulated the function of polymorphonucler leukocytes (PMN) both in vitro and in vivo. Stimulation in vitro was achieved by the incubation of PMN and HA in heparinized whole blood at concentrations of HA between 5 and 500 microgram/liter. The stimulation of the PMN function was demonstrated by an increase rate of phagocytosis of complement- and/or immunoglobulin (Ig)G-coated latex particles, increased adherence to nylon wool, increased random migration and chemotactic response, increased chemiluminescence during phagocytosis, and raised levels of intracellular ATP. The effect of HA in vivo was demonstrated, after subcutaneous administration of HA (5-20 mg) to healthy volunteers, by an enhanced rate of phagocytosis of the subsequently isolated neutrophils. The duration of the effect of one administration was approximately 1 wk with maximum effect on days 2-4. HA injections to patients with increased susceptibility to bacterial infections and impaired neutrophil function demonstrated an enhanced neutrophil function also in these individuals. HA may therefore be a new principle by which resistance to infections can be enhanced.
Full text
PDF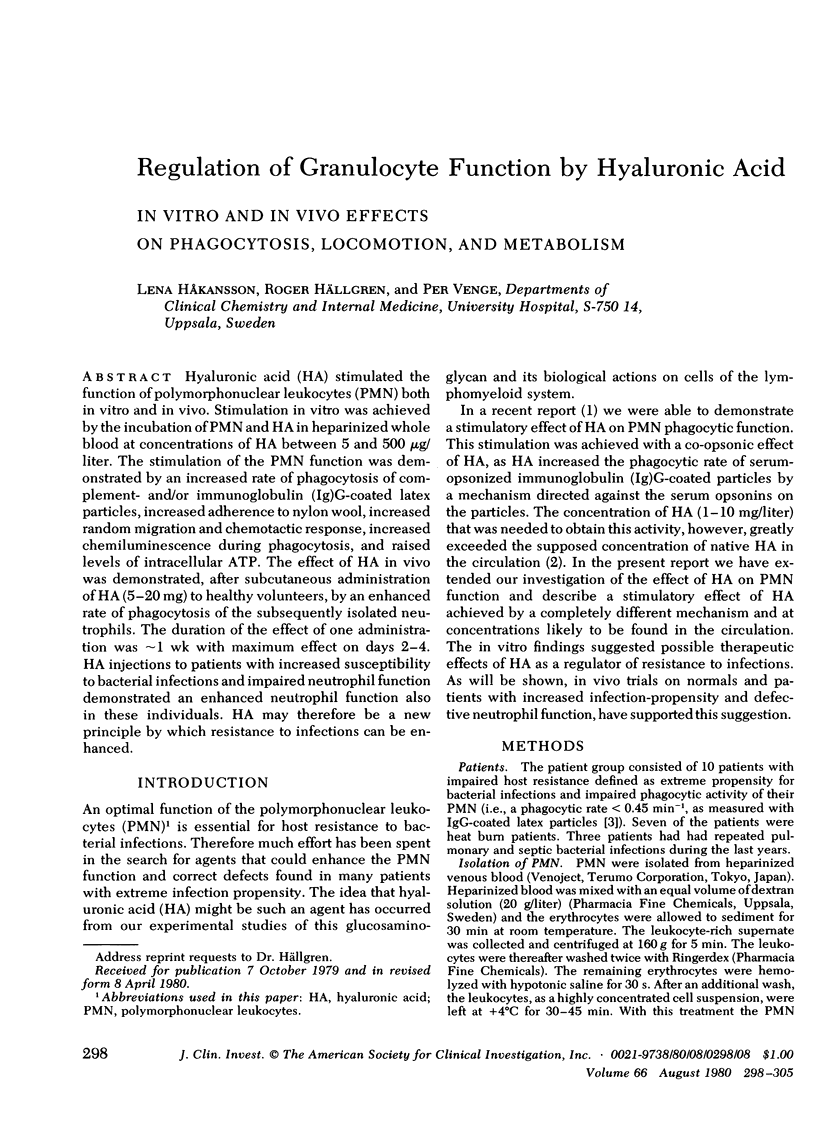


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheim A., Lindblad G. Intra-articular treatment of arthritis in race-horses with sodium hyaluronate. Acta Vet Scand. 1976;17(4):379–394. doi: 10.1186/BF03547893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs E. A., Freeman M. I., Klöti R., Meyer-Schwickerath G., Regnault F., Sweeney D. B. Hyaluronic acid and replacement of vitreous and aqueous humor. Mod Probl Ophthalmol. 1972;10:3–21. [PubMed] [Google Scholar]
- Bonventre P. F., Black-Schaffer B. The effect of neutral and acidic polysaccharides on natural resistance of mice to bacterial challenge. J Infect Dis. 1965 Oct;115(4):413–420. doi: 10.1093/infdis/115.4.413. [DOI] [PubMed] [Google Scholar]
- Brandt K. D. The effect of synovial hyaluronate on the ingestion of monosodium urate crystals by leukocytes. Clin Chim Acta. 1974 Sep 30;55(3):307–315. doi: 10.1016/0009-8981(74)90004-7. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Håkansson L., Venge P. Kinetic studies of phagocytosis. I. The serum independent particle uptake by PMN from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1978 Jan-Feb;21(1):107–113. doi: 10.1002/art.1780210117. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Jansson L., Venge P. Kinetic studies of phagocytosis of IgG-coated latex particles with a thrombocyte counter. J Lab Clin Med. 1977 Nov;90(5):786–795. [PubMed] [Google Scholar]
- Kokoshis P. L., Williams D. L., Cook J. A., Di Luzio N. R. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science. 1978 Mar 24;199(4335):1340–1342. doi: 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Murata K., Horiuchi Y. Molecular weight-dependent distribution of acidic glycosaminoglycans in human plasma. Clin Chim Acta. 1977 Feb 15;75(1):59–69. doi: 10.1016/0009-8981(77)90500-9. [DOI] [PubMed] [Google Scholar]
- Peyron J. G., Balazs E. A. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol (Paris) 1974 Oct;22(8):731–736. [PubMed] [Google Scholar]
- Venge P. Kinetic studies of cell migration in a modified Boyden chamber: dependence on cell concentration and effects of the chymotrypsin-like cationic protein of human granulocytes. J Immunol. 1979 Apr;122(4):1180–1184. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


