Abstract
Adult external genitalia (ExG) are the endpoints of normal sex differentiation. Detailed morphometric analysis and comparison of adult mouse ExG has revealed 10 homologous features distinguishing the penis and clitoris that define masculine vs. feminine sex differentiation. These features have enabled the construction of a simple metric to evaluate various intersex conditions in mutant or hormonally manipulated mice. This review focuses on the morphology of the adult mouse penis and clitoris through detailed analysis of histologic sections, scanning electron microscopy, and three-dimensional reconstruction. We also present previous results from evaluation of “non-traditional” mammals, such as the spotted hyena and wallaby to demonstrate the complex process of sex differentiation that involves not only androgen-dependent processes, but also estrogen-dependent and hormone-independent mechanisms.
Keywords: Penis, Clitoris, External genitalia, Genital tubercle, Sex differentiation
1. Introduction
Contemporary understanding of the morphogenetic and molecular mechanisms of sex differentiation of mammalian external genitalia (ExG) is still rudimentary and remains based mainly on principles enunciated by Alfred Jost over half a century ago (Jost, 1953). Jost demonstrated that masculinization of the urogenital system was dependent upon fetal testicular androgens. In the presence of androgens a male phenotype develops, whereas in the absence, the default female pathway occurs. Subsequently dihydrotestosterone (DHT) was shown to be the primary active metabolite responsible for masculinization of external genitalia in mammals (Wilson et al., 1995). This general account is supported by a wide array of clinical observations (Bin-Abbas et al., 1999; Conte et al., 1994; Ito et al., 1993). While Jost emphasized the role of androgens in masculinization of ExG, he had very little to say about the detailed anatomy of external genitalia other than two types develop, male and female. It is now apparent that male and female ExG each contain a constellation of component parts of considerable complexity and species variability (Yamada et al., 2003; Simmons and Jones, 2007).
The evolution of internal fertilization has resulted in the development of male and female organs required for this complex reproductive process. Accordingly, unique sets of male and female ExG have evolved, the penis and scrotum in males and the clitoris and vagina in females along with internal urogenital organs. Sexually dimorphic ExG have evolved in invertebrates and vertebrates (Yamada et al., 2006), and within these groups there is broad diversity of anatomical forms particularly in the penis (Simmons and Jones, 2007; Williams-Ashman, 1990). The primary penile functions are urination, copulation and sperm transfer (Simmons and Jones, 2007; Suzuki et al., 2003). Secondary penile functions include stimulation of the female and rival sperm displacement, which are achieved via morphologic specializations such as penile shape, length, papillae (spines) and other appendages (Simmons and Jones, 2007; Williams-Ashman, 1990).
Given the importance of reproduction for perpetuation of the species, sex differentiation of the ExG is an essential developmental event, which culminates in the formation of the adult penis and clitoris. Since the adult penis and clitoris are the endpoints of the process of sex differentiation, an understanding of the gross and microscopic anatomy of the normal adult penis and clitoris is essential for understanding the process of normal ExG development and its abnormalities, such as hypospadias, the most common malformation of the penis. Hypospadias occurs in approximately 1:200 to 1:300 live male births (Baskin, 2000). In the United States an unexplained doubling in the incidence of hypospadias has been reported over the last three decades (Paulozzi, 1999; Paulozzi et al., 1997). The only treatment for hypospadias is surgical repair, which can be performed successfully in the majority of patients. However, a number of patients have poor functional (may need to sit to void) and cosmetic outcomes despite multiple surgeries, in what has been described as “hypospadias cripples”. These unfortunate patients not only experience physical scarring but may also suffer from low self-esteem. Male patients with hypospadias may also have difficulty with sexuality and developing normal relationships (Baskin and Duckett, 2004). The psychological trauma to parents of newborn males with genital abnormalities must also be significant. The costs of hypospadias surgery, hospital and physician fees and time away from work to care for these young patients are a significant burden to the healthcare system.
To better understand normal development of the ExG and to prevent abnormalities such as hypospadias, it is imperative to understand sex differentiation of the ExG, its timing in appropriate animal models, the role of cell–cell interactions, the endocrine parameters of sex differentiation, morphogenesis of each component comprising the penis and clitoris, and the molecular biology of the developmental process. For many years we have had an abiding interest in development of the ExG, which has been pursued in studies of mice, moles and spotted hyenas (Yamada et al., 2003; Glickman et al., 2006; Rubenstein et al., 2003). As we have become focused specifically on the process of sex differentiation, our studies have utilized the mouse as an experimental model. First and foremost investigation of ExG development requires a detailed knowledge of the morphology of the adult mouse penis and clitoris, the endpoints of the developmental process. As we embarked upon our studies, it became apparent that the literature on the anatomy of the adult mouse penis and clitoris was both inadequate and in some cases inaccurate and certainly in need of modern morphological re-investigation. This review will focus on the updated anatomy of the adult mouse penis and the clitoris, which are the respective endpoints of masculine and feminine sex differentiation of the ExG.
2. Development of mouse genital tubercle
In mice, the penis and clitoris develop from the embryonic ambisexual genital tubercle (GT), an elevation in the fetal perineum that forms over a 4-day period (day E12–16) (Yamada et al., 2006; Perriton et al., 2002; Petiot et al., 2005). It is generally acknowledged that assembly of the ambisexual GT is complete at 16 days of gestation and that sex differentiation of the GT begins thereafter (Suzuki et al., 2002). Even though mice can be sexed visually at birth based upon anogenital distance and size of the GT (both of which are larger in males), histological examination of male and female ExG at birth reveals organ rudiments that are profoundly undifferentiated with little resemblance to the adult penis or clitoris (not illustrated). However, by 10 days postnatal sex differentiation has proceeded, and male and female ExG are profoundly different, exhibiting many of the anatomical features seen in adulthood (Rodriguez and Weiss, unpublished). Thus, sex differentiation of the ExG is initiated some time between birth and 10 days postnatal in mice. Given the importance of androgens in masculinization of the ExG (Jost, 1953; Yamada et al., 2003; Wilson et al., 1995), it is intriguing that masculine sex differentiation occurs during periods when serum androgens are particularly low (Pang et al., 1979; Pang and Tang, 1984).
3. Morphology of the adult mouse penis
In a strict sense, the adult penis is a mesoderm-derived appendage covered on its surface by ectoderm-derived skin and containing an endoderm-derived urethra. In the mouse perhaps as much as 90% of the penis is derived from mesoderm, which differentiates into erectile bodies, connective tissue, smooth muscle, bone and cartilage arranged in precise patterns required for penile function. The adult mouse penis is comprised of a body proximally and a glans distally that connect at a right angle bend (Rodriguez et al., 2011). The penile body begins as the urethra emerges from the pelvis and contains the corpus cavernosum penis and the penile urethra (Murakami, 1987). The penile glans is the segment distal to the right angle bend, and contains the penile urethra, the corpus cavernosum glandis, the MUMP (male urogenital mating protuberance), the corpus cavernosum urethrae and skeletal elements collectively called the os penis (Rodriguez et al., 2011; Murakami, 1987, 1984; Goyal et al., 2007). In conjunction with hydrostatic mechanisms in the erectile bodies, these skeletal elements presumably provide rigidity required for successful mating (Simmons and Jones, 2007; Rodriguez et al., 2011). Previous studies described the mouse and rat penis as having a blunt distal extremity with the urethral meatus at the distal tip of the penis (Murakami, 1987, 1984). Both of these observations are incorrect and have now been properly described (see below) (Rodriguez et al., 2011).
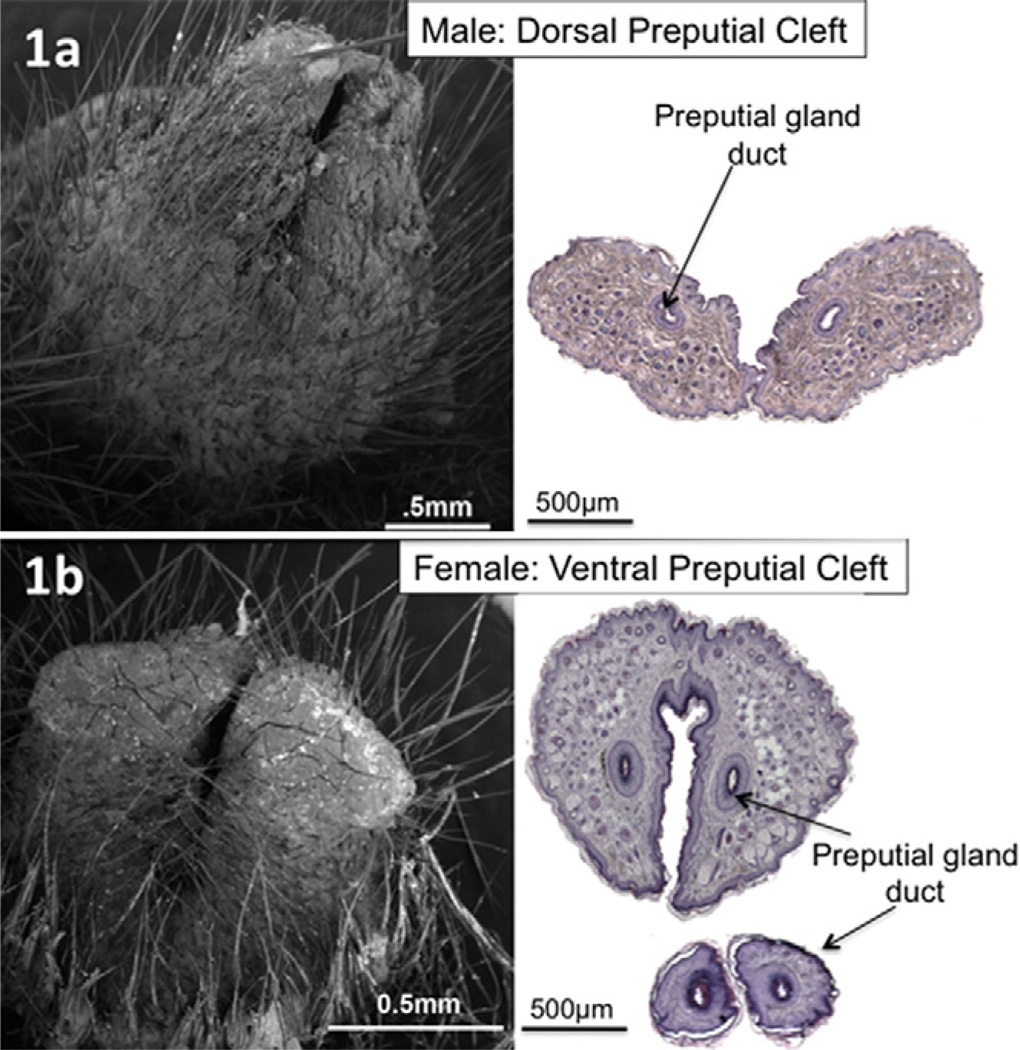
We have performed an in-depth study of the glans penis in serial transverse sections, mid-sagittal sections and by scanning electron microscopy (SEM) in order to more precisely define its morphology (Rodriguez et al., 2011; Yang et al., 2010). The surface elevation in the perineum of adult male mice is the prepuce and not the glans penis. The prepuce is bifid distally, clefted proximally, and covered with hair on its outer surface (Fig. 1a). The preputial gland ducts drain into the preputial space at the tip of each hemi-prepuce (Yang et al., 2010). The glans penis lies deep to the prepuce, and can be exposed if the prepuce is retracted.
Fig. 1.
(a) SEM of adult male penis and corresponding H&E transverse section showing distal preputial dorsal cleft. (b) SEM of adult female clitoris and corresponding H&E transverse section showing distal preputial ventral cleft. Note in the smaller section at the bottom of the figure that the preputial gland ducts are approaching their opening on the medial sides of the hemi-prepuces.
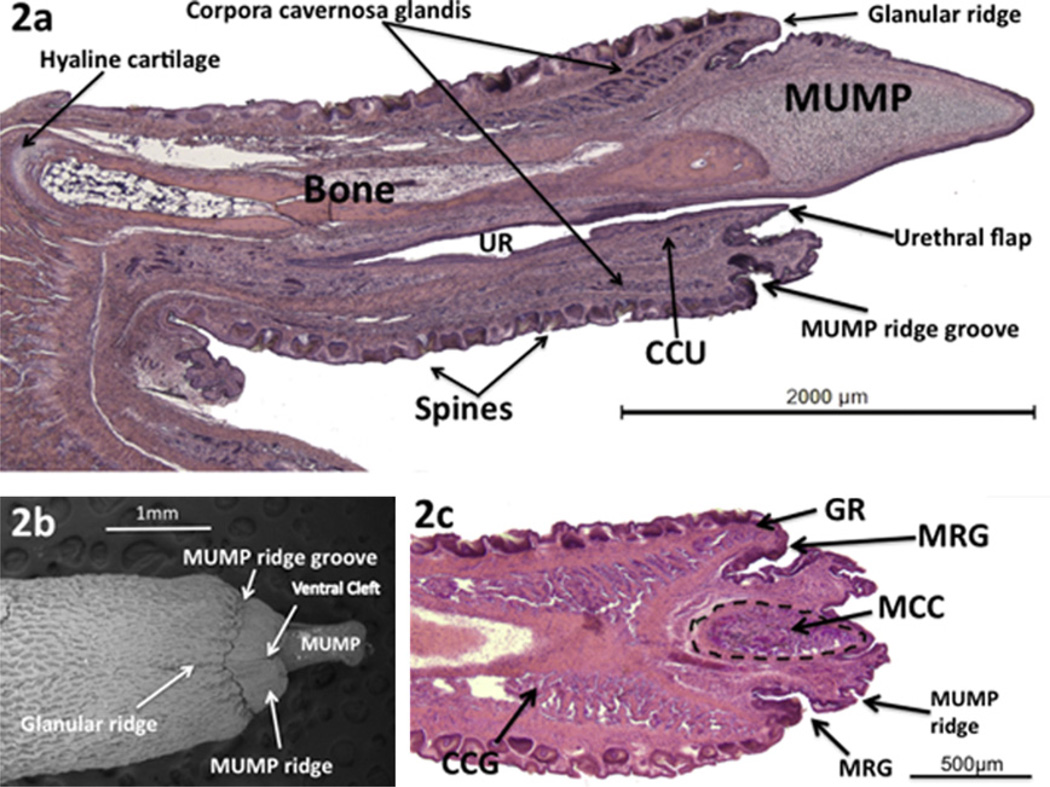
The surface of the glans penis is covered circumferentially with keratinized epithelial spines (Fig. 2a) described previously (Murakami, 1987, 1984; Iguchi et al., 1990). The distal aspect of the glans penis is adorned with a tapered projection called the male urogenital mating protuberance (MUMP) (Fig. 2a and b) (Rodriguez et al., 2011; Yang et al., 2010). The MUMP extends from the dorsal aspect of a ridge encircling the glans called the MUMP ridge (Fig. 2b and c). The MUMP ridge is defined proximally by a circumferential groove (MUMP ridge groove) (Fig. 2). The epithelium lining this groove invaginates proximally into the glans (Fig. 2a and c). Proximal to the MUMP ridge groove is another ridge encircling the glans penis called the glanular ridge (Fig. 2a–c). The MUMP ridge has a cleft in the ventral midline. This ventral cleft closes about 1 mm from the tip of the MUMP to define the urethral meatus, which is Y-shaped distally (Fig. 3) (Rodriguez et .al., 2011).
Fig. 2.
(a) Mid-sagittal H&E stained section of adult mouse penis. (b) SEM of adult mouse penis, ventral view. (c) Parasagittal H&E stained section of glans penis. MUMP = male urogenital mating protuberance; CCG = corpus cavernosumglandis; CCU = corpus cavernosum urethrae; UR = urethra; MRG = MUMP ridge groove; GR = glanular ridge; MCC = MUMP corpus cavernosum. (Modified from Rodriguez et al., 2011 with permission).
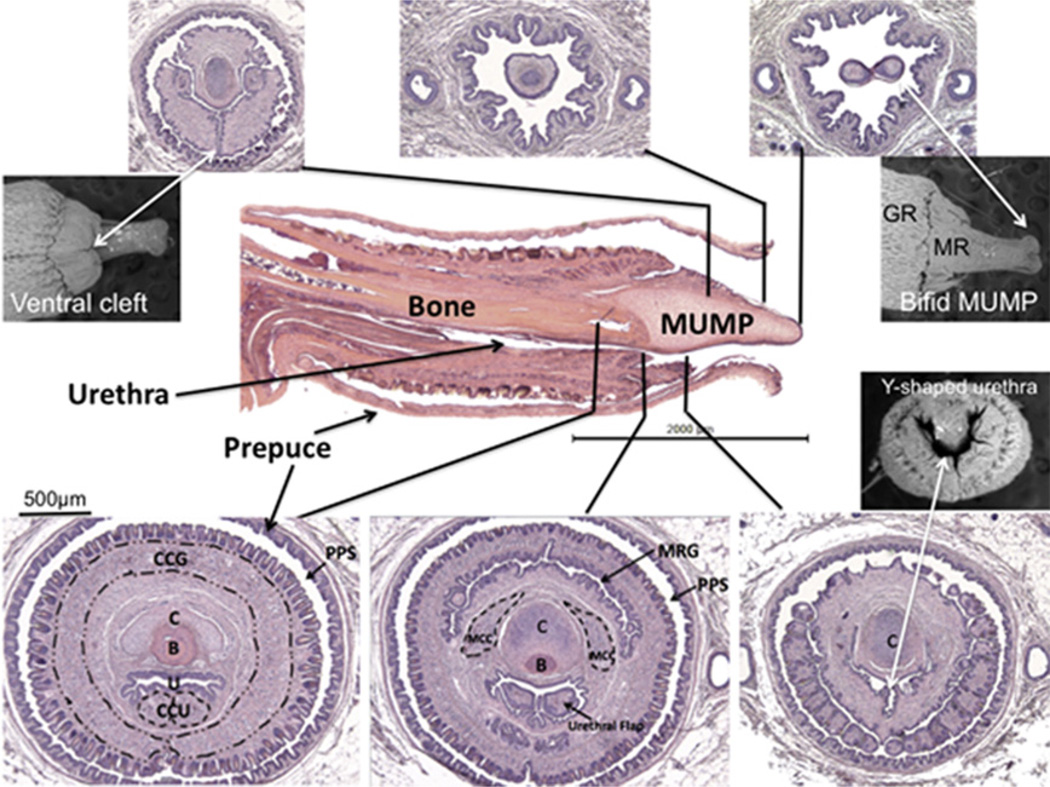
Fig. 3.
Central image: Midsagittal H&E stained section of adult male penis and surrounding H&E transverse sections. Black lines indicate exact corresponding location between transverse and longitudinal sections. Scanning electron micrographs show associated features. MUMP = male urogenital mating protuberance; CCG = corpus cavernosum glandis; CCU = corpus cavernosum urethrae; C = MUMP cartilage; B = bone; U = urethra; MRG = MUMP ridge groove; MCC = MUMP corpora cavernosa; MR: MUMP ridge; PPS = Preputial space. (Modified from Rodriguez et al., 2011 with permission).
The core element of the MUMP is fibrocartilage, which contains type I and not type II collagen (Iguchi et al., 1990). The MUMP of the adult mouse penis measures approximately 1.7 mm in length (from its distal tip to the most proximal extent of its cartilaginous core), contains a central fibrocartilagenous body and is covered by epithelium devoid of spines (Figs. 2a,b and 3) (Rodriguez et al., 2011). The MUMP ridge, MUMP ridge groove and glanular ridge seen on SEM (Fig. 2b) are well displayed in longitudinal sections (Figs. 2a,c and 3). Longitudinal sections clearly demonstrate that the urethra does not extend to the tip of the mouse penis as previously described (Murakami, 1987), but instead begins ~1 mm proximal to the distal tip of the MUMP (Figs. 2a and 3) (Rodriguez et al., 2011). This new finding may have important implications when studying hypospadias in a mouse model since urethral defects can only be ascertained in the context accurately described normal morphology. Proximal to the urethral meatus, short bilateral intra-luminal projections (urethral “flaps”) emanate from the ventral wall of the urethra and project into the urethral lumen (Rodriguez et al., 2011). These bilateral projections are untethered distally and coalesce proximally into a single structure, which attaches ventrally to the urethral wall (Fig. 3). The function of these urethral flaps is unknown.
The os penis was formally described as consisting of two components: a proximal and a distal segment. The distal segment is composed of fibrocartilage, and the proximal segment is bone. With the discovery of the MUMP, it is now more appropriate to describe the MUMP as a distal projection of the penis containing the MUMP cartilage (fibrocartilage). The actual bone should now be called the os penis, which in the adult mouse is approximately 3.8 mm in length and begins proximally near the right angle junction between the penile body and glans and extends distally to the level of the MUMP ridge groove (Rodriguez et al., 2011). Distally, the os penis is overlapped dorsally by the fibrocartilage of the MUMP (Figs. 2a and 3). The proximal aspect of the os penis consists of hyaline cartilage (Fig. 2a) expressing type II and not type I collagen as previously described (Murakami, 1987, 1984; Iguchi et al., 1990). This hyaline cartilage eventually undergoes endochondral ossification, and thus gradually disappears by approximately 6 months of age (Glucksmann et al., 1976).
The glans penis contains several discrete erectile bodies described previously (Murakami, 1987). The corpus cavernosum glandis is circumferential and extends distally into the glanular ridge in close relation to the MUMP ridge groove (Figs. 2a, c and 3). The corpus cavernosum urethrae is linear, lying ventral to the urethra (Figs. 2a and 3) as described previously (Murakami, 1987) and appears to be homologous to the corpus spongiosum of the human. We recently discovered a third set of bilateral erectile bodies (the MUMP corpora cavernosa) that flank the cartilaginous core of the MUMP within the glans penis (Rodriguez et al., 2011) (Figs. 2c and 3).
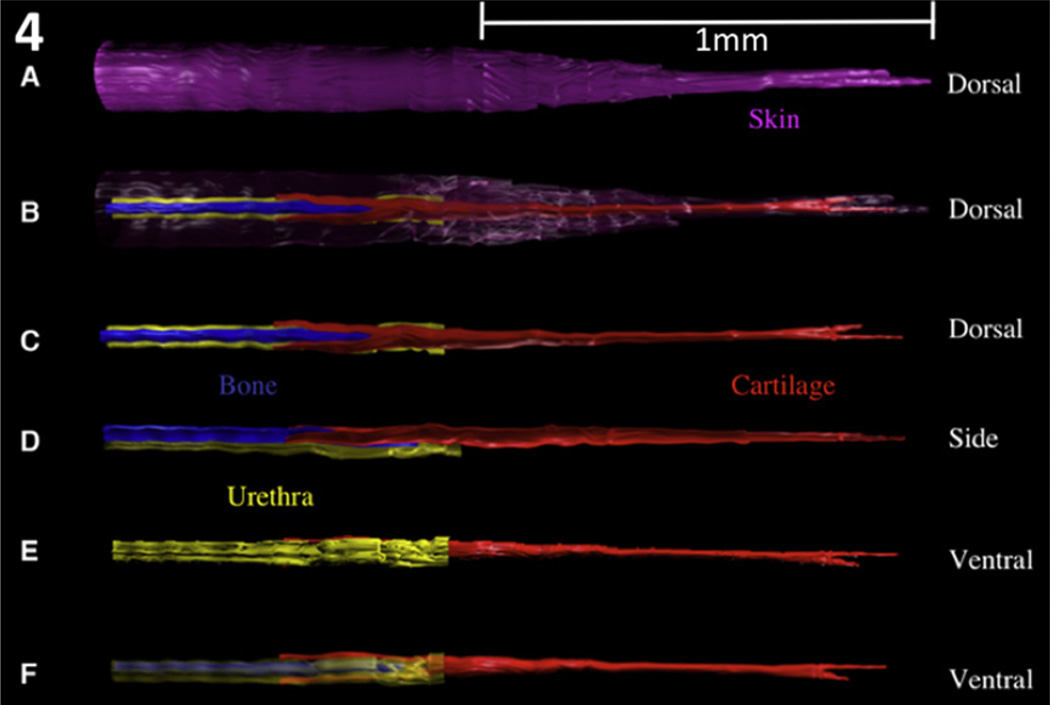
Examination of the glans penis in three different modalities (transverse sections, mid-sagittal sections and SEM) demonstrates the complex morphology and anatomic relationships of the many individual structures that comprise the glans penis of the adult mouse. By correlating the transverse sections to the corresponding levels of both the SEM and the longitudinal sections, we obtain a particularly informative perspective of proportionate sizes, location and orientation of the penile elements (Fig. 3). To further appreciate spatial relationships, 3D reconstructions were prepared from 7 µm serial section sets (Fig. 4), which reinforce the information derived from the transverse and longitudinal sections and the SEMs. The MUMP fibrocartilage extends proximally into the glans to dorsally overlap the os penis. Both the cartilage and bone lie dorsal to the penile urethra. As stated above the tubular urethra begins ~1 mm proximal to the distal tip of the MUMP, where the edges of the ventral cleft fuse. In total there are at least 10 homologous morphological features that distinguish the adult mouse penis from the adult clitoris enumerated in Table 1 and illustrated in the figures (Rodriguez et al., 2011).
Fig. 4.
Three-dimensional reconstructions of the adult mouse glans penis. The components are not to scale as they have been elongated to better demonstrate 3D relationships. Figures (a–c) are dorsal views showing that the MUMP cartilage is bifid distally and overlaps the bone proximally. Figures (d–f) are side and ventral views that demonstrate cartilage and bone lying dorsal to the penile urethra. Note the tubular urethra does not extend to the distal tip of the penis. In B the skin has been rendered semitransparent. In F the urethra has been rendered semitransparent. Purple = skin; red = cartilage; blue = bone; yellow = urethra. (With permission, Rodriguez et al. 2011).
Table 1.
Homologous features: male and female adult external genitalia.
| Male | Female | ||
|---|---|---|---|
| Large organ width (~2020 µm) | 1 | Small organ width (~740 µm) | 0 |
| Defined erectile bodies | 1 | Diffuse erectile tissue | 0 |
| Distal fibrocartilage | 1 | No distal fibrocartilage | 0 |
| Proximal hyaline cartilage | 1 | No proximal hyaline cartilage | 0 |
| Keratinized epithelial spines | 1 | No epithelial spines | 0 |
| Long bone (~3800 µm) | 1 | Short bone (~580 µm) | 0 |
| Circumferential penile epithelium | 1 | U-shaped clitoral epithelium | 0 |
| No tethering, freely mobile | 1 | Ventral tethering, immobile | 0 |
| Dorsal cleft | 1 | Ventral cleft | 0 |
| Urethra completely within penis | 1 | Urethra never entirely within clitoris | 0 |
| Total score WT male | 10 | Total score WT female | 0 |
4. Morphology of the adult mouse clitoris
Since ExG of the adult mice represent endpoints of the process of sex differentiation in which the ambisexual genital tubercle develops into either a penis or clitoris, the genesis of the morphological differences between these organs becomes paramount in understanding the process of sex differentiation of the ExG. Unfortunately, previous descriptions of the adult mouse clitoris lack sufficient detail to fully understand the anatomy and histology of this important organ of female sexual response. Accordingly, to better elucidate the anatomy of the adult mouse clitoris, we examined the ExG of adult female CD-1 (n = 3), C57BL/6 (n = 5), and αERKONorth Carolina (n = 4) mice and of Tfm/Y (n = 3) “male” mice by serial section histology, scanning electron microscopy, and three-dimensional reconstruction, techniques used previously to describe the adult mouse penis (Rodriguez et al., 2011). We define dorsal and ventral in the female according to the penile convention. Ventral is the preputial surface closest to the anus or the vagina, and dorsal is the surface most furthest from the anus.
The adult female perineum contains the prepuce, the clitoris, the urethra and the vaginal ostium. The external elevation in the female mouse perineum is not the clitoris, but rather is the prepuce (Yang et al., 2010). In both sexes, the penis and clitoris lie deep (internal) to the prepuce. The homology of the female prepuce to that of the male is unmistakable. SEM analysis reveals that the female prepuce is hair-bearing externally, bifid distally, and is ventrally clefted (Fig. 1b). Serial H&E sections confirm the bifid and clefted nature, and demonstrates that the ducts of the preputial glands open on the distal-most medial surfaces of the two halves of the prepuce similar to that seen in the male (Fig. 1b). The level where the ventral preputial cleft fuses defines the urethral meatus (Fig. 5a). At the urethral meatus the lumen is elongated in the dorsal–ventral plane (Fig. 5a), but more proximally it assumes a redundant star-shaped form (Fig. 5c–f).
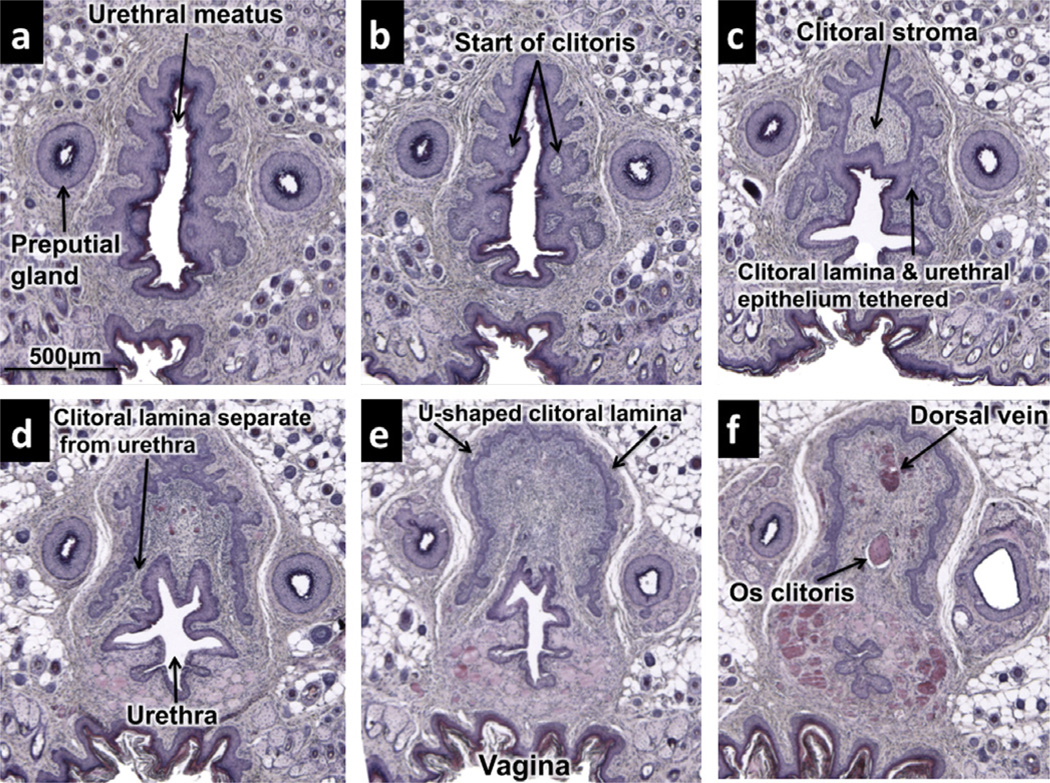
Fig. 5.
Transverse histologic sections of adult clitoris beginning distally (a) and progressing sequentially proximally (b–f).(a) Beginning of urethra at level where preputial cleft closes. (b) Distal tip of clitoris is first visualized as small “stromal islands” within a complex central epithelium defining the urethral lumen. (c) Clitoral epithelial lamina defining the clitoral stroma (arrows) is still connected to urethral epithelium. (d) U-shaped clitoral lamina is now separate from urethral epithelium. (e) Well-formed Ushaped clitoral epithelial lamina. Urethra still is partly within the U-shaped epithelial lamina at this level. (f) Os clitoris visible dorsal to urethra. Urethra is completely outside of epithelial lamina of clitoris at this level.
The clitoris of the adult mouse is an organ defined in large part by a U-shaped epithelial lamina (Fig. 5d–f), which is characteristic of the “body of the clitoris” (Martin-Alguacil et al., 2008). At approximately 750 µm from the distal tip of the prepuce, small lightly H&E-stained masses of stroma can be seen within the morphologically complex central epithelium surrounding the urethral lumen (Fig. 5b). This stroma is the distal-most extent of the clitoris and is located just proximal to the urethral meatus. Initially the central epithelium defining the urethra and the distal aspect of the clitoris are in continuity (Fig. 5c), but proximally the central epithelium separates into a defined urethra and U-shaped clitoral lamina (Fig. 5d–f). Distally, the urethra lies in part within the space defined by the U-shaped clitoral lamina (Fig. 5c–e). However, proximally the urethra takes a ventral course and eventually lies completely outside (ventral) of the clitoral lamina (Fig. 5f).
An os clitoris (Fig. 5f) was present in adult female CD-1, C57BL/6 mice, and aERKO mice, as well as in “male” X/YTfm mice, which have a clitoris almost identical to that of a normal female mouse. The os clitoris is a slender minuscule bone measuring only ~581 µm in length in adult female CD-1 and C57BL/6 mice and only ~185 µm in X/YTfm mice. In contrast, it is much broader and longer in αERKO female mice, measuring ~1840 µm in length. The presence of an os clitoris in all of the above three mouse strains suggests that its formation is independent of signaling through both the androgen receptor and estrogen receptor alpha.
Many small blood vessels are present within the clitoral stroma enclosed within the U-shaped clitoral lamina. Proximally a prominent single midline blood vessel (dorsal vein) is observed (Fig. 5F). Small blood vessels within the clitoris are not organized into defined erectile bodies as in the male, but instead exhibit a diffuse pattern.
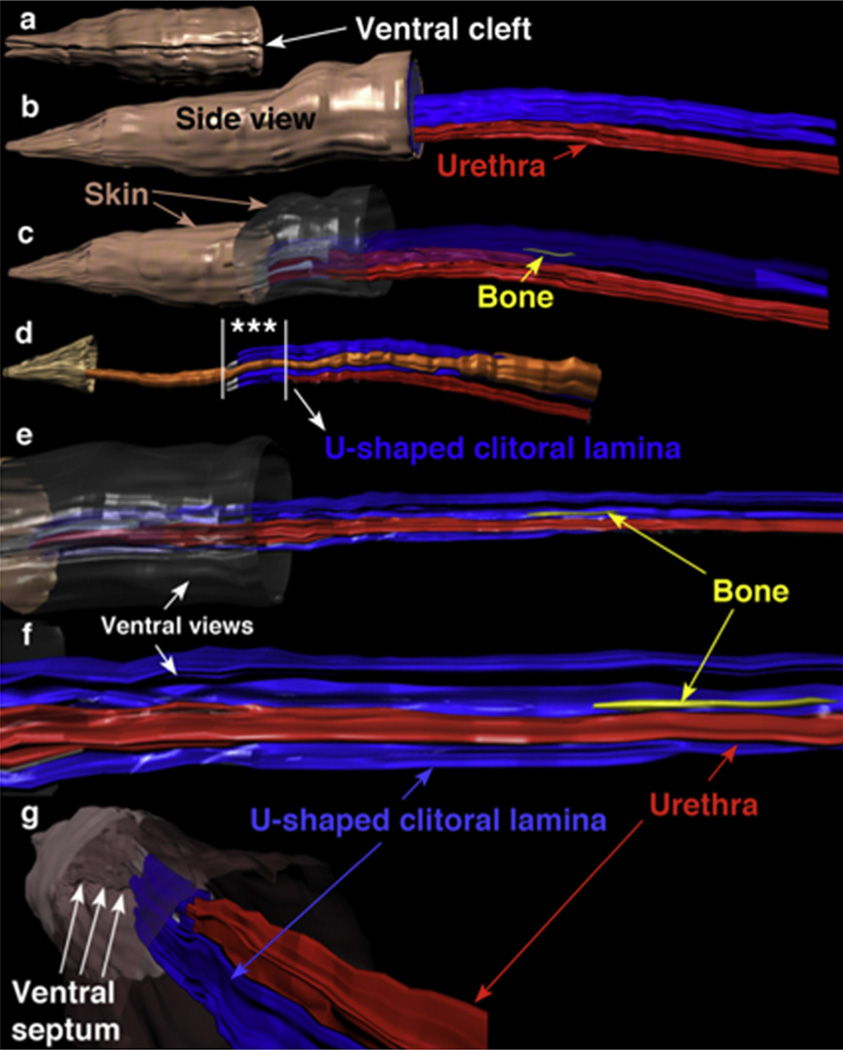
Three-dimensional reconstruction allows for precise demonstration of the spatial relationships between the individual elements that compose the adult female mouse ExG. The female prepuce is ventrally clefted and covered by hair (Figs. 1b and Fig. 6a). Side views (Fig. 6b–d) with or without the skin rendered semitransparent or invisible and ventral views (Fig. 6e–g) demonstrate that the os clitoris lies within the concavity of the U-shaped clitoral lamina and is located dorsal to the urethra. In Fig. 6d the preputial gland ducts can be seen in their lateral position. Note the asterisks in Fig. 6d, which denote the distal end of the clitoris and urethra where both structures share a common central epithelium as seen in histological sections (Fig. 5b and c).
Fig. 6.
Three-dimensional reconstructions of the adult mouse clitoris, which has been artificially elongated for clarity. (a) Ventral view of the distal portion of the female prepuce showing the ventral cleft, which is in proportional register with the side views (b–d). (b) Side view with entire skin (brown) opaque showing clitoral lamina (blue) dorsal to the urethra (red). (c) Side view with distal skin opaque and proximal skin and U-shaped clitoral lamina semi-transparent. These modifications allow visualization of the distal aspect of the clitoris and the os clitoris. (d) Side view with most of the skin invisible showing preputial gland ducts (orange) and urethra ventral to clitoral lamina. Asterisks between the vertical white lines denote the distal aspect of the clitoris where both the clitoris and urethra share a common central epithelium as seen in histological sections (Fig. 5b and c). (e) Ventral view into the concavity of the U-shaped clitoral lamina tilted slightly to be able to see the os clitoris (yellow). Compare with Fig. 6c to see that the os clitoris is within the concavity of the U-shaped clitoral lamina. (f) Higher magnification of (e). (g) View from a proximal perspective looking distally into skin tube showing the ventral cleft and the ventral septum (arrows). Fusion of the ventral edges of the cleft creates the urethral meatus as demonstrated in Fig. 5a. Note that distal clitoris abuts the ventral lamina.(For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Discussion
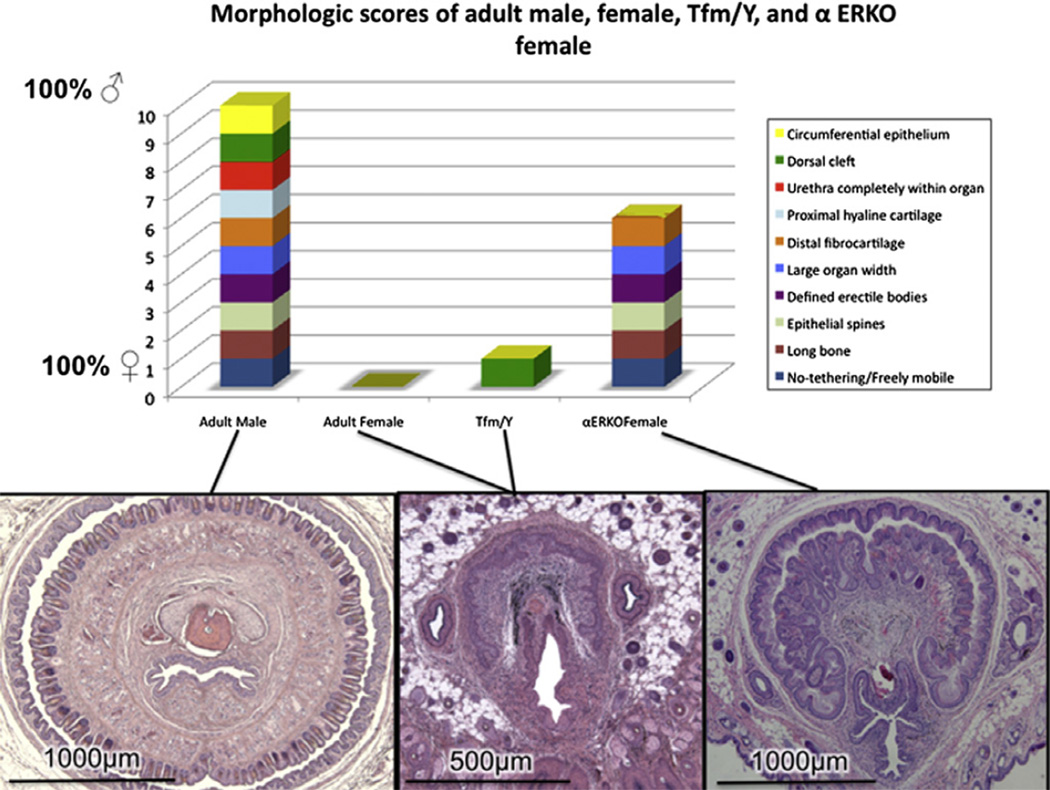
Since the penis and clitoris arise from a common precursor, the fetal GT, and are homologous organs, homologies exist between the individual components comprising these organs. These homologies are illustrated in Table 1 and Fig. 7 in which all male features are ascribed the value of 1, and all homologous female features are given a value of zero. Accordingly, objective comparative measures can be performed on ExG of adult wild-type male mice which score 10, and adult wild-type female mice which score zero (Rodriguez et al., 2011). This approach now enables an objective morphometric analysis of male and female ExG of wild-type, mutant or hormonally manipulated mice. Using a similar approach it should be possible to construct a metric for assessing homologous male and female ExG features during sex differentiation at any given stage of development.
Fig. 7.
Morphologic scores of adult male, female, αERKO female and X/YTfm “male” mice. Note male scores 10, female scores 0, αERKO females are partially masculinized with score of 6, and X/YTfm “male” mice score 1, very similar to normal female mice (Modified from Yang et al. (2010) with permission).
Several morphological differences distinguish the adult penis from the adult clitoris, which represent endpoints of the process of sex differentiation. One such distinguishing feature between the clitoris and glans penis is mobility. The glans clitoris is not contained within a circumferential epithelial lined preputial space, but instead is tethered (attached to surrounding tissues) along its entire length. Note in the region of the U-shaped clitoral lamina, the stroma of the glans clitoris is confluent ventrally with non-clitoral stroma (Fig. 5d–f). Conversely, the glans penis lies free within a circumferential epithelial lined preputial space, and accordingly is untethered and mobile. During mating (and presumably urination) the penis projects outward beyond the prepuce. Another feature distinguishing the clitoris from the penis is erectile tissue, which is well defined anatomically in the penis (Rodriguez et al., 2011; Murakami, 1987) and diffuse in the clitoris. Other distinguishing features include: organ size (small for the clitoris and large for the penis); bone (small for the clitoris and large for the penis); distal fibrocartilage and proximal hyaline cartilage (absent in the clitoris and present the penis), surface spines (absent in the clitoris and present the penis); urethral position (never completely within the clitoris versus completely within the penis); crosssectional shape (U-shaped for the clitoris and circular for the penis) (Table 1). These features distinguishing the normal adult mouse clitoris and penis serve as an important baseline for comparing mutant strains of mice which may exhibit a range of sexual ambiguities involving one or more of these distinguishing homologous features. In this regard, X/YTfm mice, which are profoundly feminized due to the absence of functional androgen receptors, express a spectrum of features characteristic of a normal clitoris. When ExG of adult female αERKONorth Carolina mice is subjected to morphometric analysis, the masculinization reported earlier (Yang et al., 2010) scores six on the adult ExG metric scale (Fig. 7). A similar metric applied to mutant and hormonally manipulated mice will provide the tools for future exploration of the role of estrogens, growth factors and transcription factors in sex differentiation of the ExG.
Based upon several observations, it has been suggested that estrogens (in addition to androgens) are critical factors in normal development of male ExG. Androgen receptors (AR) have been detected in the embryonic GT (Suzuki et al., 2002; Murakami, 1987) and adult penis (Schultheiss et al., 2003). Estrogen receptors alpha and beta (ERα and ERβ) and aromatase have been detected in the developing rat penis (Jesmin et al., 2002, 2004). ERα and/or ERβ have also been detected in adult and fetal human penile tissue (Crescioli et al., 2003; Dietrich et al., 2004), fetal mouse penis and clitoris (Agras et al., 2007), and in adult mouse clitoris (Martin-Alguacil et al., 2008). Ligands (testosterone and estradiol) for these receptors are present in serum at low levels during the neonatal period when sex differentiation of the ExG is occurring. Significantly, developing ExG express AR, ERα and ERβ and aromatase, and are exposed to both testosterone and estradiol in the neonatal period when sex differentiation is occurring. We presume that certain features within developing ExG are responsive/dependent upon signaling through AR, ERα and ERβ for normal sex differentiation and that normal sex differentiation results from the sum of signaling through androgen and estrogen receptors. The challenge will be to identify specific morphogenetic processes dependent upon signaling through each of these receptors (AR, ERα and ERβ) and interactions between these signaling systems.
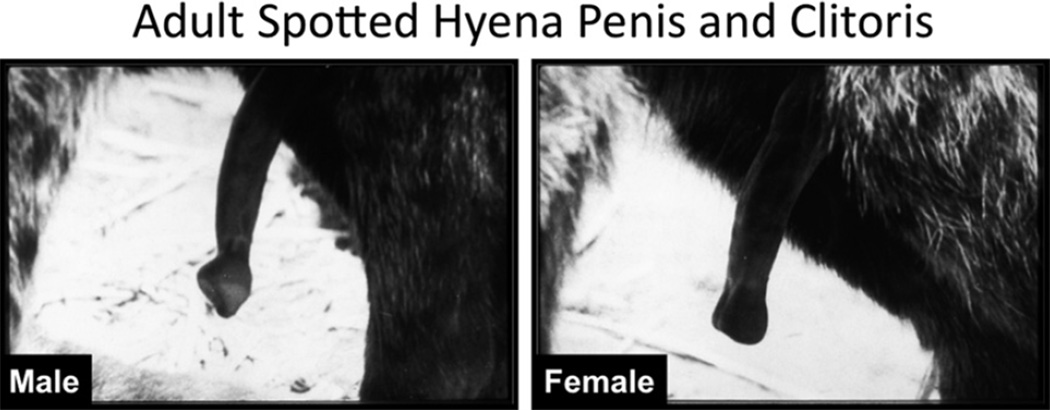
Finally, detailed morphometric analysis as described above may reveal other mechanisms involved in the development of the ExG. For example, hormone-independent mechanisms may occur during development of the ExG, as in the case of the spotted hyena. Female spotted hyenas are the only extant mammals that mate and give birth through a penile clitoris (Fig. 8), which is similar in size and shape to the penis and is traversed by a central canal. Female spotted hyenas also have a pseudoscrotum, but no external vaginal os. It is understood that androgens are required for masculine development of mammalian ExG. For example, androgens are essential for fusion of the urethral folds to form a penile urethra and elongation of the phallus (Yamada et al., 2003). Are androgens driving the formation of the peniform clitoris in the spotted hyena? To explore this possibility, our studies of the spotted hyena were initiated in 1985 to search for naturally circulating androgens at critical stages in the development of the female spotted hyena that would account for the “masculinization” of their ExG. From our studies we determined that the hyena placenta produces testosterone via placental conversion of maternal androstenedione to testosterone from an early stage of gestation (Licht et al., 1992; Yalcinkaya et al., 1993). However, placental androgens were not responsible for formation of the large phallus in the female spotted hyena. This conclusion emerged from studies in which pregnant hyenas were treated with a cocktail of anti-androgens (flutamide and finasteride). These anti-androgen studies revealed developmental events that were androgen-dependent and those, which were androgen-independent. Natural sex differences in the hyena phallus at mid-shaft include: (a) position of the retractor muscles (ventral in males and dorsal in females), (b) size and shape of the urogenital sinus/urethra (small and oval in males and large and pleated in females), (c) the tunica albuginea (which surrounds the corporal body and urethra in males but surrounds the corporal body only in females), (d) corpus spongiosum (present in males and absent in females) (Cunha et al., 2003, 2005). These sex differences emerge and are consolidated between 45 and 65-days of gestation during differentiation of Leydig cells in the fetal testes and the appearance of steroidogenic enzymes (Browne et al., 2006). All of the above male features at mid-shaft are reversed to the female pattern by anti-androgens in utero (Cunha et al., 2005). Conversely, female features can be masculinized by in utero treatment with the synthetic androgen, mibolerone (Place, Wilson and Cunha, unpublished). In 45-day and 65-day fetal hyena ExG androgen receptors were detected by immunohistochemistry in the corporal body, retractor muscles, and the lining of the urethra/urogenital sinus (Cunha et al., 2005). Thus, androgen blockade was effective in inhibiting prostatic development and “feminizing” the above internal structures (a–d above) at midshaft in the penis (change from penile to clitoral patterning) (Cunha et al., 2005; Forger et al., 1996). However, overall phallic length remained substantial with only a 15–20% reduction in males and females as a result of anti-androgen treatment (Drea et al., 1998, 2002). Therefore, formation of an elongated phallus containing a urethra appears to be an androgen-independent event in male and female spotted hyenas (Cunha et al., 2005), even though certain features at midshaft in the phallus are androgen-dependent. In a separate experiment postnatal growth of the phallus of male and female spotted hyenas was investigated by gonadectomizing pups at 6 months of age and measuring phallic length in intact and gonadectomized animals at two years. Castration at 6 months had no effect on penile growth out to 2 years, while ovariectomy at 6 months only modestly reduced clitoral length (Glickman et al., 1998) suggesting that growth of the phallus in male and female spotted hyenas is hormone-independent, which is consistent with the in utero anti-androgen studies.
Fig. 8.
Adult spotted hyena external genitalia. Note that the clitoris is of similar size as that of the penis even though the morphology of the glans is different in the two sexes (Modified from Frank et al. (1990) with permission).
6. Conclusion
While Jost’s theory of ExG development focusing exclusively on the presence or absence of androgen action is undoubtedly correct, it neglects that possibility of other endocrine mechanisms such as estrogen action and relegates female development as a default pathway, which discouraged search for active processes involved in the development of female ExG (Drea et al., 1999). We emphasize that normal female sex differentiation of the ExG is a unique active process and not simply a default pathway associated with absence of androgen. Taking a broader view through examination of “non-traditional” mammals such as in the wallaby, spotted hyena, certain moles and lemurs has changed/enriched our view of sexual differentiation through demonstration of hormone-independent mechanisms in development of ExG (Cunha et al., 2005; Wilson et al., 2002; Renfree and Short, 1988). Presence of ERa, ERb and aromatase in developing ExG as well as estrogen ligands in serum when sex differentiation is occurring emphasizes the potential importance of estrogenic mechanisms in ExG development. Finally, signaling via AR and ERα can be antagonistic at cellular, tissue and systemic levels, and thus the balance between AR and ER signaling may be critical in normal male and female development of the ExG.
Acknowledgments
Supported by NSF Grant IOS-0920793, NIH grant RO1 DK0581050 and NH&MRC grant APP1002733.
Abbreviations
- ExG
external genitalia
- GT
genital tubercle
- DHT
dihydrotestosterone
- SEM
scanning electron microscopy
- MUMP
male urogenital mating protuberance
- ERKO
estrogen receptor alpha knockout
- AR
androgen receptor
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
References
- Agras K, Willingham E, Shiroyanagi Y, Minasi P, Baskin LS. Estrogen receptor-alpha and beta are differentially distributed, expressed and activated in the fetal genital tubercle. Journal of Urology. 2007;177:2386–2392. doi: 10.1016/j.juro.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Baskin LS. Hypospadias and urethral development. Journal of Urology. 2000;163:951–956. [PubMed] [Google Scholar]
- Baskin LS, Duckett JW. Hypospadias: long-term outcome. In: Mouriquand P, editor. Long-Term Outcome Pediatric Surgery. Philadelphia: Saunders; 2004. pp. 563–564. [Google Scholar]
- Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. The Journal of Pediatrics. 1999;134:579–583. doi: 10.1016/s0022-3476(99)70244-1. [DOI] [PubMed] [Google Scholar]
- Browne P, Place NJ, Vidal JD, Moore IT, Cunha GR, Glickman SE, Conley AJ. Endocrine differentiation of fetal ovaries and testes of the spotted hyena (Crocuta crocuta): timing of androgen-independent versus androgen-driven genital development. Reproduction. 2006;132:649–659. doi: 10.1530/rep.1.01120. [DOI] [PubMed] [Google Scholar]
- Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom) The Journal of Clinical Endocrinology and Metabolism. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. Expression of functional estrogen receptors in human fetal male external genitalia. Journal of Clinical Endocrinology and Metabolism. 2003;88:1815–1824. doi: 10.1210/jc.2002-021085. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wang Y, Place NJ, Liu W, Baskin L, Glickman SE. Urogenital system of the spotted hyena (Crocuta crocuta Erxleben): a functional histological study. Journal of Morphology. 2003;256:205–218. doi: 10.1002/jmor.10085. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Place NJ, Baskin L, Conley A, Weldele M, Cunha TJ, Wang YZ, Cao M, Glickman SE. The ontogeny of the urogenital system of the spotted hyena (Crocuta crocuta Erxleben) Biology of Reproduction. 2005;73:554–564. doi: 10.1095/biolreprod.105.041129. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Haitel A, Huber JC, Reiter WJ. Expression of estrogen receptors in human corpus cavernosum and male urethra. Journal of Histochemistry and Cytochemistry. 2004;52:355–360. doi: 10.1177/002215540405200306. [DOI] [PubMed] [Google Scholar]
- Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. Journal of Reproduction and Fertility. 1998;113:117–127. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- Drea C, Coscia E, Glickman S. Hyenas. In: Knobil E, et al., editors. Encyclopedia of Reproduction. San Diego: Academic Press; 1999. pp. 718–724. [Google Scholar]
- Drea CM, Place NJ, Weldele ML, Coscia EM, Licht P, Glickman SE. Exposure to naturally circulating androgens during foetal life incurs direct reproductive costs in female spotted hyenas, but is prerequisite for male mating. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2002;269:1981–1987. doi: 10.1098/rspb.2002.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurone in spotted hyenas. Journal of Comparative Neurology. 1996;375:333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Frank L, Glickman S, Powch I. Sexual dimorphism in the spotted hyaena (Crocuta crocuta) Journal of Zoology London. 1990;221:308–313. [Google Scholar]
- Glickman S, Coscia E, Frank L, Licht P, Weldele M, Drea C. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 3. Effects of juvenile gonadectomy. Journal of Reproduction and Fertility. 1998;113:129–135. doi: 10.1530/jrf.0.1130129. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Cunha GR, Drea CM, Conley AJ, Place NJ. Mammalian sexual differentiation: lessons from the spotted hyena. Trends in Endocrinology and Metabolism. 2006;17:349–356. doi: 10.1016/j.tem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Glucksmann A, Ooka-Souda S, Miura-Yasugi E, Mizuno T. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. Journal of Anatomy. 1976;121:363–370. [PMC free article] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Williams JW. Role of estrogen in induction of penile dysmorphogenesis: a review. Reproduction. 2007;134:199–208. doi: 10.1530/REP-07-0207. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Irisawa S, Uesugi Y, Kusunoki S, Takasugi N. Abnormal development of the os penis in male mice treated neonatally with tamoxifen. Acta Anatomica. 1990;139:201–208. doi: 10.1159/000146998. [DOI] [PubMed] [Google Scholar]
- Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, Sakuma I, Hattori Y, Kitabatake A. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology. 2002;143:4764–4774. doi: 10.1210/en.2002-220628. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Sakuma I, Matsuda N, Togashi H, Yoshioka M, Hattori Y, Kitabatake A. Aromatase is abundantly expressed by neonatal rat penis but downregulated in adulthood. Journal of Molecular Endocrinology. 2004;33:343–359. doi: 10.1677/jme.1.01548. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Progress in Hormone Research. 1953;8:379–418. doi: 10.1016/b978-1-4831-9825-5.50017-8. [DOI] [PubMed] [Google Scholar]
- Licht P, Frank LG, Pavgi S, Yalcinkaya TM, Siiteri PK, Glickman SE. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 2. maternal and fetal steroids. Journal of Reproduction and Fertility. 1992;95:463–474. doi: 10.1530/jrf.0.0950463. [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Pfaff DW, Kow LM, Schober JM. Oestrogen receptors and their relation to neural receptive tissue of the labia minora. BJU International. 2008;101:1401–1406. doi: 10.1111/j.1464-410X.2008.07626.x. [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Schober J, Kow LM, Pfaff D. Oestrogen receptor expression and neuronal nitric oxide synthase in the clitoris and preputial gland structures of mice. BJU International. 2008;102:1719–1723. doi: 10.1111/j.1464-410X.2008.07989.x. [DOI] [PubMed] [Google Scholar]
- Murakami R. Histogenesis of the os penis and os clitoridis in rats. Development, Growth and Differentiation. 1984;26:419–426. doi: 10.1111/j.1440-169X.1984.00419.x. [DOI] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wildtype and androgen-insensitive mice. Journal of Anatomy. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Murakami R. Autoradiographic studies of the localisation of androgenbinding cells in the genital tubercles of fetal rats. Journal of Anatomy. 1987;151:209–219. [PMC free article] [PubMed] [Google Scholar]
- Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. Journal of Endocrinology. 1984;100:7–11. doi: 10.1677/joe.0.1000007. [DOI] [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL, Pang CS. Serum concentrations of testosterone, oestrogens, luteinizing hormone and folliclestimulating hormone in male and female rats during the critical period of neural sexual differentiation. Journal of Endocrinology. 1979;80:103–110. doi: 10.1677/joe.0.0800103. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environmental Health Perspectives. 1999;107:297–302. doi: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–834. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Developmental Biology. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- Rodriguez EDAW, Jr, Yang JH, Menshenina J, Ferretti M, Cunha T, Barcellos D, Chan LY, Risbridger G, Cunha GR, Baskin LS. New insights on the morphology of adult mouse penis. Biology of Reproduction. 2011 doi: 10.1095/biolreprod.111.091504. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein NM, Cunha GR, Wang YZ, Campbell KL, Conley AJ, Catania KC, Glickman SE, Place NJ. Variation in ovarian morphology in four species of new world moles with a peniform clitoris. Reproduction. 2003;126:713–719. doi: 10.1530/rep.0.1260713. [DOI] [PubMed] [Google Scholar]
- Schultheiss D, Badalyan R, Pilatz A, Gabouev AI, Schlote N, Wefer J, von Wasielewski R, Mertsching H, Sohn M, Stief CG, Jonas U. Androgen and estrogen receptors in the human corpus cavernosum penis: immunohistochemical and cell culture results. World Journal of Urology. 2003;21:320–324. doi: 10.1007/s00345-003-0371-y. [DOI] [PubMed] [Google Scholar]
- Simmons MN, Jones JS. Male genital morphology and function: an evolutionary perspective. Journal of Urology. 2007;177:1625–1631. doi: 10.1016/j.juro.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ogino Y, Murakami R, Satoh Y, Bachiller D, Yamada G. Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evolution and Development. 2002;4:133–141. doi: 10.1046/j.1525-142x.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw 3rd EB, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman HG. Enigmatic features of penile development and functions. Perspectives in Biology and Medicine. 1990;33:335–374. doi: 10.1353/pbm.1990.0008. [DOI] [PubMed] [Google Scholar]
- Wilson JD, George FW, Renfree MB. The endocrine role in mammalian sexual differentiation. Recent Progress in Hormone Research. 1995;50:349–364. doi: 10.1016/b978-0-12-571150-0.50021-4. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Shaw G, Leihy ML, Renfree MB. The marsupial model for male phenotypic development. Trends in Endocrinology and Metabolism. 2002;13:78–83. doi: 10.1016/s1043-2760(01)00525-2. [DOI] [PubMed] [Google Scholar]
- Yalcinkaya TM, Siiteri PK, Vigne JL, Licht P, Pavgi S, Frank LG, Glickman SE. A mechanism for virilization of female spotted hyenas in utero. Science. 1993;260:1929–1931. doi: 10.1126/science.8391165. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Developmental Dynamics. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. Journal of Urology. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]