Abstract
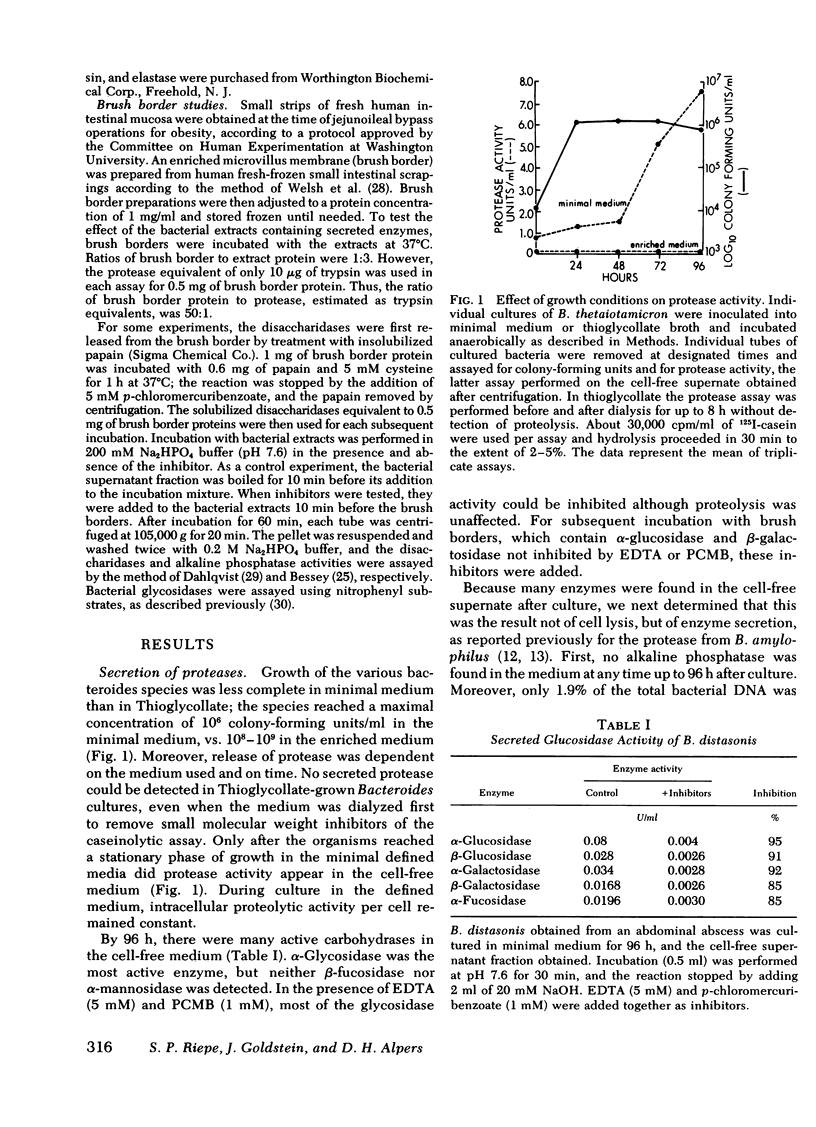
Selected bacteroides species secreted various amounts of protease and glycosidase into their growth medium. Bacteroides vulgatus, distasonis, and ovatus secreted the most (31-60% of total). The secreted protease was similar in action to the protease within the organism, in that it had a broad pH optimum of 6-9, a Km app. for casein of 0.1 μM, and was inhibited by benzamidine, phenylmethylsulfonyl fluoride, diisopropylfluorophosphate (DIFP), and by an elastase inhibitor, Ac(Ala)3AlaCH2Cl.
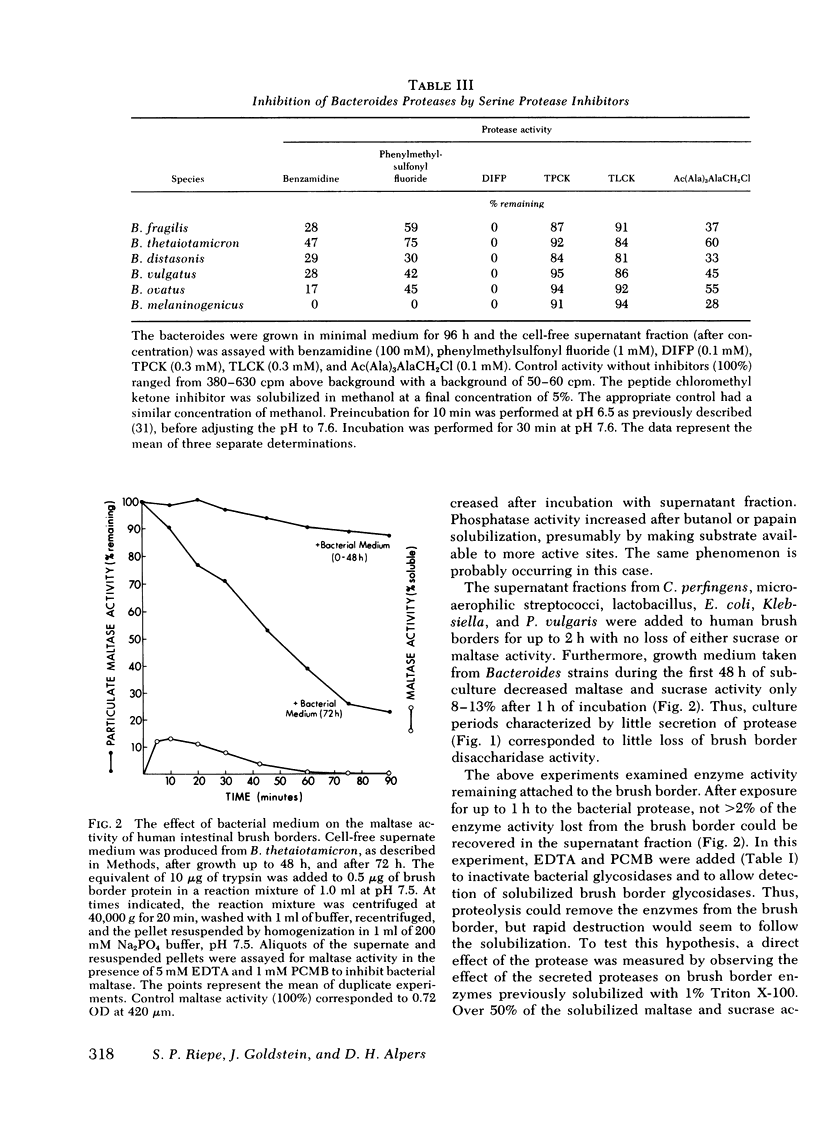
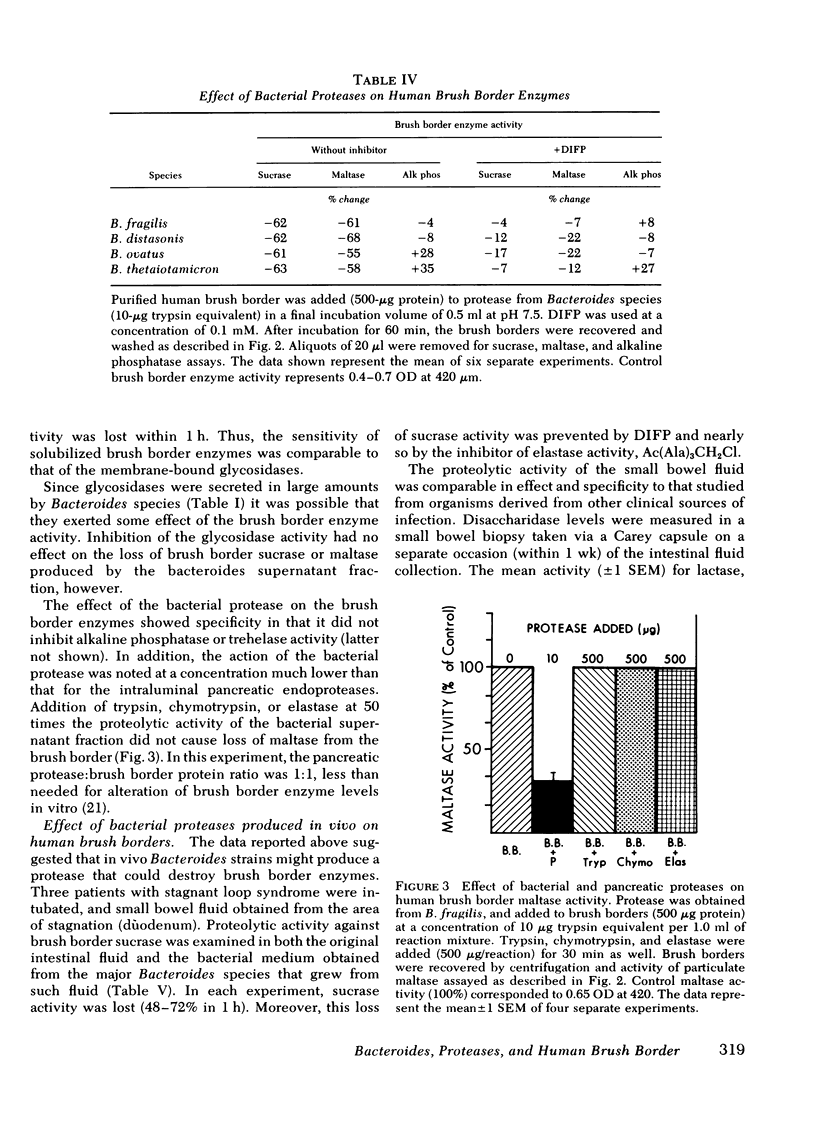
Exposure of human brush border preparations to the secreted protease reduced maltase and sucrase activities; the reduction could be prevented by DIFP. In contrast, brush border alkaline phosphatase activity either did not change or increased after exposure to bacterial secretions. >90% inhibition of secreted glycosidase using EDTA and p-chloromercuribenzoic acid did not prevent the reduction of brush border maltase and sucrase activity, suggesting that glucosidases were not likely to be involved in the destruction of brush border enzymes. Moreover, the bacterial proteases caused only a small net release of active maltase or sucrase from the brush border. Most of the loss of activity was due to destruction of the enzyme. Proximal bowel fluid of three patients with overgrowth contained DIFP-inhibitable protease that destroyed sucrase in isolated brush borders. A Bacteroides species was isolated from each sample that secreted protease and destroyed brush border sucrase. We conclude that in bacterial overgrowth syndromes, brush border damage may occur from protease(s) secreted by Bacteroides.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Tedesco F. J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim Biophys Acta. 1975 Aug 5;401(1):28–40. doi: 10.1016/0005-2736(75)90338-7. [DOI] [PubMed] [Google Scholar]
- Arvanitakis C., Olsen W. A. Intestinal mucosal disaccharidases in chronic pancreatitis. Am J Dig Dis. 1974 May;19(5):417–421. doi: 10.1007/BF01255605. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn T. H. The protease liberated from Bacteroides amylophilus strain H18 by mechanical disintegration. J Gen Microbiol. 1968 Aug;53(1):37–51. doi: 10.1099/00221287-53-1-37. [DOI] [PubMed] [Google Scholar]
- Bloch R., Menge H., Lorenz-Meyer H., Stöckert H. G., Riecken E. O. Functional, biochemical and morphological alterations in the intestines of rats with an experimental blind-loop syndrome. Res Exp Med (Berl) 1975 Nov 26;166(1):67–78. doi: 10.1007/BF01851347. [DOI] [PubMed] [Google Scholar]
- Brandt L. J., Bernstein L. H., Wagle A. Production of vitamin B 12 analogues in patients with small-bowel bacterial overgrowth. Ann Intern Med. 1977 Nov;87(5):546–551. doi: 10.7326/0003-4819-87-5-546. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am J Clin Nutr. 1974 Nov;27(11):1313–1319. doi: 10.1093/ajcn/27.11.1313. [DOI] [PubMed] [Google Scholar]
- Coello-Ramirez P., Lifshitz F. Enteric microflora and carbohydrate intolerance in infants with diarrhea. Pediatrics. 1972 Feb;49(2):233–242. [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Drasar B. S., Shiner M. Studies on the intestinal flora. II. Bacterial flora of the small intestine in patients with gastrointestinal disorders. Gut. 1969 Oct;10(10):812–819. doi: 10.1136/gut.10.10.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G. Release of intestinal surface-membrane glycoproteins associated with enzyme activity by brief digestion with papain. Biochem J. 1971 Mar;121(5):781–789. doi: 10.1042/bj1210781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Toskes P. P. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974 Nov;67(5):965–974. [PubMed] [Google Scholar]
- Gracey M., Houghton M., Thomas J. Deoxycholate depresses small-intestinal enzyme activity. Gut. 1975 Jan;16(1):53–56. doi: 10.1136/gut.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M., Papadimitriou J., Bower G. Ultrastructural changes in the small intestines of rats with self-filling blind loops. Gastroenterology. 1974 Oct;67(4):646–651. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haenel H. Human normal and abnormal gastrointestinal flora. Am J Clin Nutr. 1970 Nov;23(11):1433–1439. doi: 10.1093/ajcn/23.11.1433. [DOI] [PubMed] [Google Scholar]
- Hamilton J. D., Dyer N. H., Dawson A. M., O'Grady F. W., Vince A., Fenton J. C., Mollin D. L. Assessment and significance of bacterial overgrowth in the small bowel. Q J Med. 1970 Apr;39(154):265–285. [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S. Degradation of bile salts by human intestinal bacteria. Gut. 1968 Feb;9(1):22–27. doi: 10.1136/gut.9.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C. Bacterial degradation of gastrointestinal mucins. II. Bacterial origin of fecal ABH(O) blood group antigen-destroying enzymes. Gastroenterology. 1968 Feb;54(2):218–224. [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- Jonas A., Flanagan P. R., Forstner G. G. Pathogenesis of mucosal injury in the blind loop syndrome. Brush border enzyme activity and glycoprotein degradation. J Clin Invest. 1977 Dec;60(6):1321–1330. doi: 10.1172/JCI108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A., Krishnan C., Forstner G. Pathogenesis of mucosal injury in the blind loop syndrome. Gastroenterology. 1978 Nov;75(5):791–795. [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Production of protease and elastase by Pseudomonas aeruginosa strains isolated from patients. Infect Immun. 1977 Mar;15(3):679–685. doi: 10.1128/iai.15.3.679-685.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKINSON T. M., OLSON J. A. Inhibitory effects of bile acids on the uptake, metabolism, and transport of water-soluble substances in the small intestine of the rat. Life Sci. 1963 Jun;6:393–398. doi: 10.1016/0024-3205(63)90123-1. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. Biochemistry. 1973 Nov 6;12(23):4767–4774. doi: 10.1021/bi00747a032. [DOI] [PubMed] [Google Scholar]
- Prizont R., Hersh T., Floch M. H. Jejunal bacterial flora in chronic small bowel disease. I. Celiac disease. II. Regional enteritis. Am J Clin Nutr. 1970 Dec;23(12):1602–1607. doi: 10.1093/ajcn/23.12.1602. [DOI] [PubMed] [Google Scholar]
- Regnier P., Thang M. N. Subcellular distribution and characterization of endo and exo-cellular proteases in E. coli. Biochimie. 1972;54(10):1227–1236. doi: 10.1016/s0300-9084(72)80063-4. [DOI] [PubMed] [Google Scholar]
- Rogers A. I., Rothman S. L. Blind loop syndrome. Postgrad Med. 1974 Apr;55(4):99–105. doi: 10.1080/00325481.1974.11713734. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. H., Hardison W. G., Bull D. M. Abnormal bile-salt patterns and intestinal bacterial overgrowth associated with malabsorption. N Engl J Med. 1967 Jun 22;276(25):1391–1397. doi: 10.1056/NEJM196706222762501. [DOI] [PubMed] [Google Scholar]
- Seetharam B., Grimme N., Goodwin C., Alpers D. H. Differential sensitivity of intestinal brush border enzymes to pancreatic and lysosomal proteases. Life Sci. 1976 Jan 1;18(1):89–95. doi: 10.1016/0024-3205(76)90278-2. [DOI] [PubMed] [Google Scholar]
- Swaminathan N., Radhakrishnan A. N. Characterization of two hetero-beta-galactosidases from monkey small intestine. Arch Biochem Biophys. 1969 Dec;135(1):288–295. doi: 10.1016/0003-9861(69)90542-6. [DOI] [PubMed] [Google Scholar]
- Sykes P. A., Boulter K. H., Schofield P. F. The microflora of the obstructed bowel. Br J Surg. 1976 Sep;63(9):721–725. doi: 10.1002/bjs.1800630913. [DOI] [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Welsh J. D., Preiser H., Woodley J. F., Crane R. K. An enriched microvillus membrane preparation from frozen specimens of human small intestine. Gastroenterology. 1972 Apr;62(4):572–582. [PubMed] [Google Scholar]


