Abstract
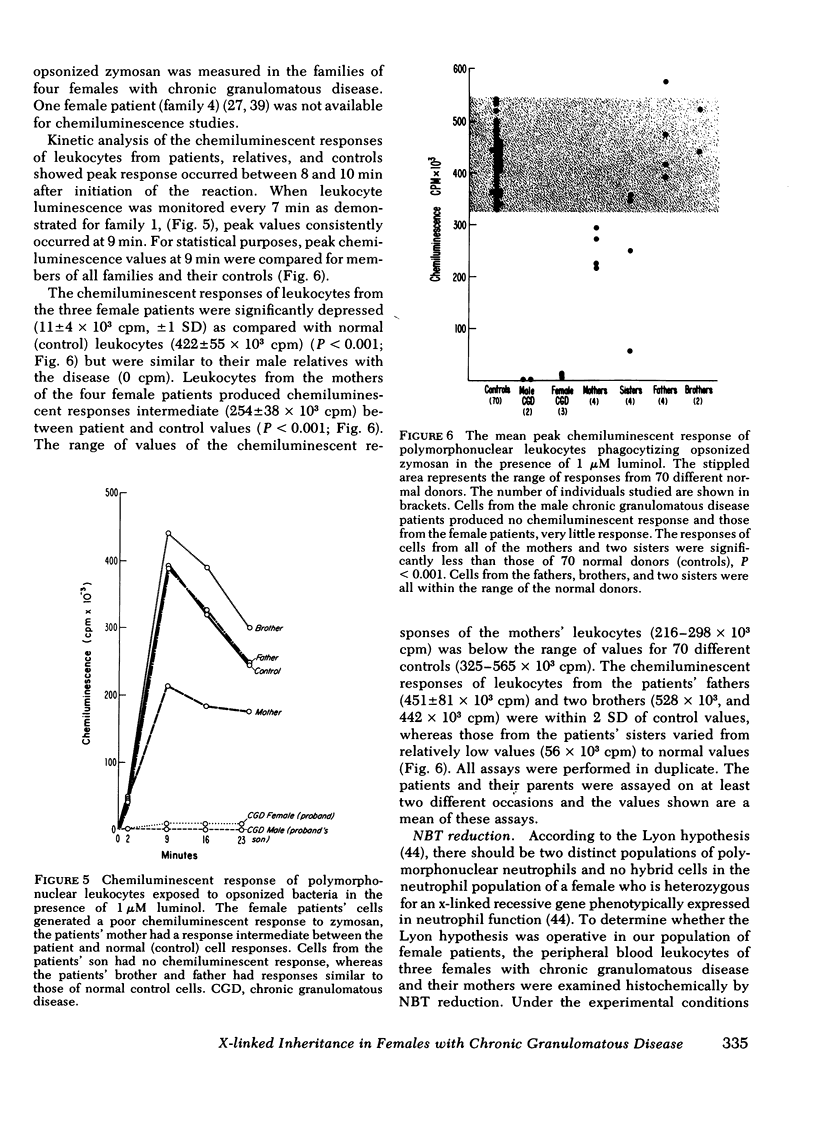
Chronic granulomatous disease in males is familial and its transmission is is usually clearly x-linked. The mode of inheritance in females with the syndrome is unknown and the carrier state difficult to identify. Defective polymorphonuclear leukocyte bactericidal activity in this disease is associated with an absence of the respiratory burst generated in stimulated phagocytes and may be detected by the chemiluminescence assay. Polymorphonuclear leukocytes from three of four females with chronic granulomatous disease had extremely low chemiluminescence production, their asymptomatic mothers had intermediate values, and their fathers were normal. Polymorphonuclear neutrophils of two affected males in these kinships generated no chemiluminescence, whereas two of seven female relatives had intermediate values, and all nonaffected males had normal values. In the three families in which leukocytes were studied by nitroblue tetrazolium reduction, two populations of neutrophils were demonstrated for the female patients and/or their mothers. The wide phenotypic variability for clinical disease, evidence of two leukocyte populations in the patients or their mothers, and low but detectable leukocyte chemiluminescence in the affected females is consistent with the Lyon hypothesis of x-chromosome inactivation in these families. The findings suggest an x-linked inheritance in these females with chronic granulomatous disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Reed M. A., Harper T. B., 3rd, Gupta S., Steele R. H., Waring W. W. Correlation of metabolic and chemiluminescent responses of granulocytes from three female siblings with chronic granulomatous disease. J Infect Dis. 1977 Oct;136(4):510–518. doi: 10.1093/infdis/136.4.510. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Azimi P. H., Bodenbender J. G., Hintz R. L., Kontras S. B. Chronic granulomatous disease in three female siblings. JAMA. 1968 Dec 23;206(13):2865–2870. [PubMed] [Google Scholar]
- BERENDES H., BRIDGES R. A., GOOD R. A. A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn Med. 1957 May;40(5):309–312. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Biggar W. D., Buron S., Holmes B. Chronic granulomatous disease in an adult male: A proposed X-linked defect. J Pediatr. 1976 Jan;88(1):63–70. doi: 10.1016/s0022-3476(76)80728-7. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Lundmark K. M. Abnormal nitroblue tetrazolium test in relatives of a female with chronic granulomatous disease. Scand J Infect Dis. 1972;4(2):167–169. doi: 10.3109/inf.1972.4.issue-2.20. [DOI] [PubMed] [Google Scholar]
- Carruthers J. A., Greaves M. W. Chronic granulomatous disease. Br J Dermatol. 1976 Jul;95 (Suppl 14):72–74. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F. A., Klebanoff S. J. Chronic granulomatous disease: studies of a family with impaired neutrophil chemotactic, metabolic and bactericidal function. Am J Med. 1978 Dec;65(6):941–948. doi: 10.1016/0002-9343(78)90745-3. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Huntley C. C., Muss H. B., Bass D. A. Oxidative metabolism of the human eosinophil. Blood. 1977 Sep;50(3):525–535. [PubMed] [Google Scholar]
- Dupree E., Smith C. W., MacDougall N. L., Long W. K., Goldman A. S. Undetected carrier state in chronic granulomatous disease. J Pediatr. 1972 Oct;81(4):770–774. doi: 10.1016/s0022-3476(72)80100-8. [DOI] [PubMed] [Google Scholar]
- Griscom N. T., Kirkpatrick J. A., Jr, Girdany B. R., Berdon W. E., Grand R. J., Mackie G. G. Gastric antral narrowing in chronic granulomatous disease of childhood. Pediatrics. 1974 Oct;54(4):456–460. [PubMed] [Google Scholar]
- Harvath L., Amirault H. J., Andersen B. R. Chemiluminescence of human and canine polymorphonuclear leukocytes in the absence of phagocytosis. J Clin Invest. 1978 May;61(5):1145–1154. doi: 10.1172/JCI109029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Andersen B. R. Defective initiation of oxidative metabolism in polymorphonuclear leukocytes. N Engl J Med. 1979 May 17;300(20):1130–1135. doi: 10.1056/NEJM197905173002003. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Kaliner M. Immunologic and nonimmunologic generation of superoxide from mast cells and basophils. J Clin Invest. 1978 Jan;61(1):187–196. doi: 10.1172/JCI108917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Newman S. L. Chronic granulomatous disease. Pediatr Clin North Am. 1977 May;24(2):365–376. doi: 10.1016/s0031-3955(16)33424-1. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Durack D. T., Rosen H., Clark R. A. Functional studies on human peritoneal eosinophils. Infect Immun. 1977 Jul;17(1):167–173. doi: 10.1128/iai.17.1.167-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Kontras S. B., Bass J. C. Chronic granulomatous disease. Lancet. 1969 Sep 20;2(7621):646–647. doi: 10.1016/s0140-6736(69)90358-4. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- LANDING B. H., SHIRKEY H. S. A syndrome of recurrent infection and infiltration of viscera by pigmented lipid histiocytes. Pediatrics. 1957 Sep;20(3):431–438. [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S., Wilfert C., Johnston R. B., Jr, McCall C. E. Deficiency of NADPH oxidase activity in chronic granulomatous disease. J Pediatr. 1977 Feb;90(2):213–217. doi: 10.1016/s0022-3476(77)80632-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Shin H., Goya N., Nakagawara A. Identification of a carrier mother of a female patient with chronic granulomatous disease. J Pediatr. 1976 Nov;89(5):784–786. doi: 10.1016/s0022-3476(76)80807-4. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Igo R. P. The NBT slide test: a simple screening method for detecting chronic granulomatous disease and female carriers. J Pediatr. 1973 Jul;83(1):77–82. doi: 10.1016/s0022-3476(73)80316-6. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Kaplan E. L., Page A. R., Gruskay F. L., Malawista S. E. Defective polymorphonuclear-leukocyte function and chronic granulomatous disease in two female children. N Engl J Med. 1968 May 2;278(18):976–980. doi: 10.1056/NEJM196805022781802. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C., White J. G., Holmes B. Spectrum of function of neutrophils from carriers of sex-linked chronic granulomatous disease. J Pediatr. 1975 Dec;87(6 Pt 1):901–907. doi: 10.1016/s0022-3476(75)80902-4. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T., Webster D., Allison A. C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978 Aug 26;2(8087):446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder J. S., Schorr W. F. Primary disseminated pulmonary aspergillosis with metastatic skin nodules. Successful treatment with inhalation nystatin therapy. JAMA. 1969 Aug 25;209(8):1191–1195. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H., Larson W. M., Webb D. I. Letter: NBT results in chronic granulomatous disease. J Pediatr. 1974 Feb;84(2):311–312. doi: 10.1016/s0022-3476(74)80647-5. [DOI] [PubMed] [Google Scholar]
- Windhorst D. B., Page A. R., Holmes B., Quie P. G., Good R. A. The pattern of genetic transmission of the leukocyte defect in fatal granulomatous disease of childhood. J Clin Invest. 1968 May;47(5):1026–1034. doi: 10.1172/JCI105792. [DOI] [PMC free article] [PubMed] [Google Scholar]