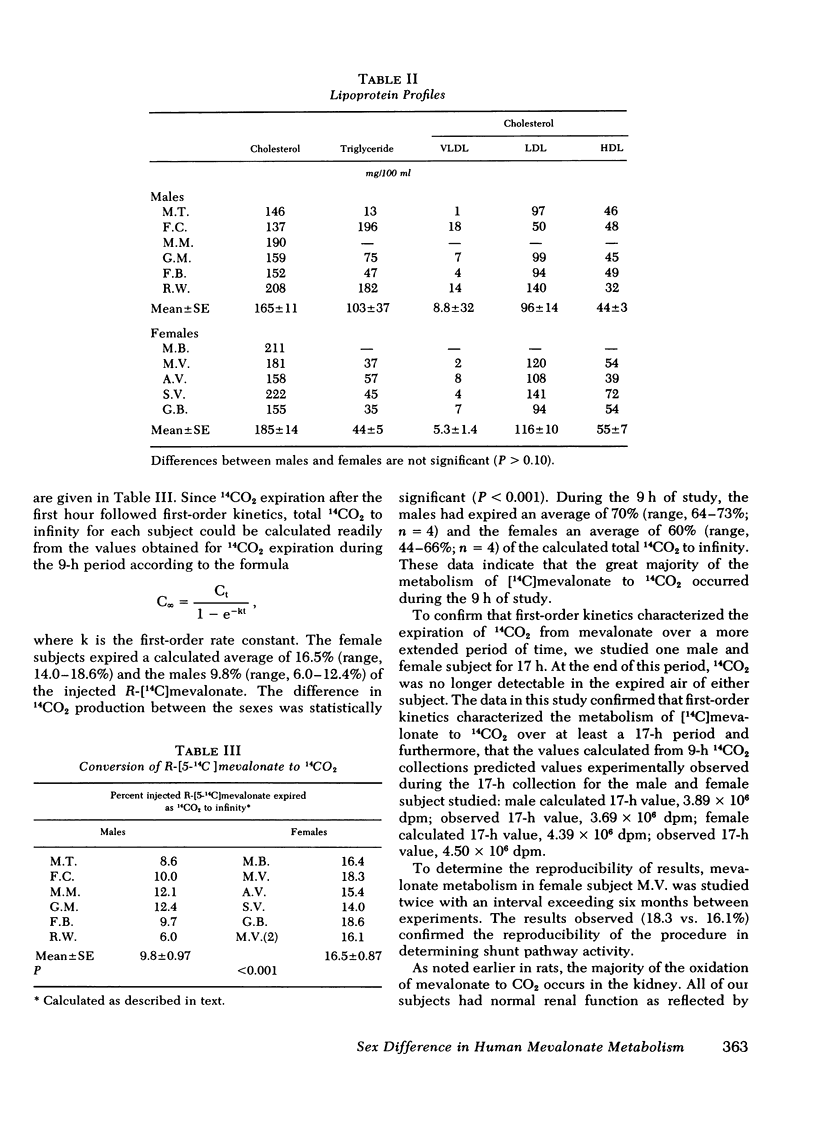
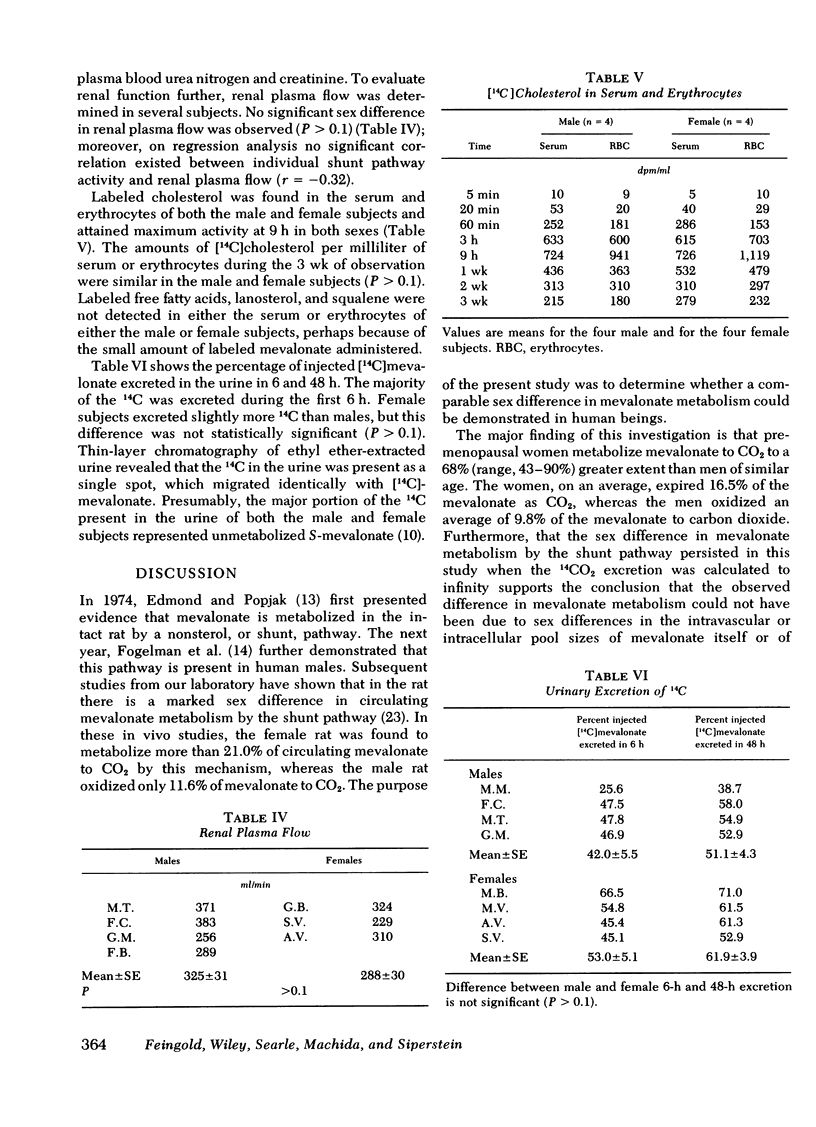
Abstract
Two pathways of mevalonate metabolism have been demonstrated: the major (sterol) pathway leads to cholesterol synthesis, whereas the second shunts mevalonate away from sterol production and ultimately results in its oxidation to CO2. Previous studies have demonstrated that the female rat metabolizes circulating mevalonate by the shunt pathway at twice the rate of the male, whereas the male rat converts significantly more circulating mevalonate to cholesterol than the female. The present study extends these observations to humans. Six men and five premenopausal women with normal renal function were injected with R,S-[5-14C]mevalonate, and 14CO2 expired in the breath of the subjects was monitored continuously with an ionization chamber. On an average, the female subjects expired 16.5% and the males 9.8% of the injected R-[5-14C]mevalonate (P less than 0.001). No differences were observed in the plasma and erythrocyte [14C]cholesterol levels. These data demonstrate, in human beings, a sex difference in mevalonate metabolism. The overall impact of the greater mevalonate shunt activity on cholesterol balance in women is unknown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth P. H., Draper H. H., Hemming F. W., Morton R. A. In vivo incorporation of [2-14-C]-mevalonate into dolichol of rabbit and pig liver. Arch Biochem Biophys. 1966 Mar;113(3):646–653. doi: 10.1016/0003-9861(66)90243-8. [DOI] [PubMed] [Google Scholar]
- Edmond J., Fogelman A. M., Popják G. Mevalonate metabolism: role of kidneys. Science. 1976 Jul 9;193(4248):154–156. doi: 10.1126/science.935865. [DOI] [PubMed] [Google Scholar]
- Edmond J., Popják G. Transfer of carbon atoms from mevalonate to n-fatty acids. J Biol Chem. 1974 Jan 10;249(1):66–71. [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Popjåk G. Metabolism of mevalonate in rats and man not leading to sterols. J Biol Chem. 1975 Mar 10;250(5):1771–1775. [PubMed] [Google Scholar]
- GARATTINI S., PAOLETTI P., PAOLETTI R. Lipid biosynthesis in vivo from acetate-1-C 14 and 2-C 14 and mevalonic-2-C 14 acid. Arch Biochem Biophys. 1959 Sep;84:253–255. doi: 10.1016/0003-9861(59)90579-x. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S., AVIGAN J., STEINBERG D. Studies of cholesterol biosynthesis. V. The time course and pathway of the later stages of cholesterol biosynthesis in the livers of intact rats. J Biol Chem. 1963 Apr;238:1287–1293. [PubMed] [Google Scholar]
- Gold P. H., Olson R. E. Studies on coenzyme Q. The biosynthesis of coenzyme Q9 in rat tissue slices. J Biol Chem. 1966 Aug 10;241(15):3507–3516. [PubMed] [Google Scholar]
- Gough D. P., Hemming F. W. The characterization and stereochemistry of biosynthesis of dolichols in rat liver. Biochem J. 1970 Jun;118(1):163–166. doi: 10.1042/bj1180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom K. H., Siperstein M. D., Bricker L. A., Luby L. J. Studies of the in vivo metabolism of mevalonic acid in the normal rat. J Clin Invest. 1973 Jun;52(6):1303–1313. doi: 10.1172/JCI107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C. The effect of cholesterol feeding and fasting upon beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1967 Mar 10;242(5):990–993. [PubMed] [Google Scholar]
- Popják G., Boehm G., Parker T. S., Edmond J., Edwards P. A., Fogelman A. M. Determination of mevalonate in blood plasma in man and rat. Mevalonate "tolerance" tests in man. J Lipid Res. 1979 Aug;20(6):716–728. [PubMed] [Google Scholar]
- Raskin P., Siperstein M. D. Mevalonate metabolism by renal tissue in vitro. J Lipid Res. 1974 Jan;15(1):20–25. [PubMed] [Google Scholar]
- Righetti M., Wiley M. H., Murrill P. A., Siperstein M. D. The in vitro metabolism of mevalonate by sterol and non-sterol pathways. J Biol Chem. 1976 May 10;251(9):2716–2721. [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- WAGONER R. D., TAUXE W. N., MAHER F. T., HUNT J. C. MEASUREMENT OF EFFECTIVE RENAL PLASMA FLOW WITH SODIUM IODOHIPPURATE I-131. JAMA. 1964 Mar 14;187:811–813. doi: 10.1001/jama.1964.03060240019004. [DOI] [PubMed] [Google Scholar]
- White L. W., Rudney H. Regulation of 3-hydroxy-3-methylglutarate and mevalonate biosynthesis by rat liver homogenates. Effects of fasting, cholesterol feeding, and triton administration. Biochemistry. 1970 Jun 23;9(13):2725–2731. doi: 10.1021/bi00815a021. [DOI] [PubMed] [Google Scholar]
- Wiley M. H., Howton M. M., Siperstein M. D. Sex differences in the sterol and nonsterol metabolism of mevalonate. J Biol Chem. 1979 Feb 10;254(3):837–842. [PubMed] [Google Scholar]
- Wiley M. H., Howton M. M., Siperstein M. D. The quantitative role of the kidneys in the in vivo metabolism of mevalonate. J Biol Chem. 1977 Jan 25;252(2):548–554. [PubMed] [Google Scholar]
- Wiley M. H., Murrill P. A., Howton M. M., Huling S. L., Cohen D. C., Siperstein M. D. The role of kidneys in mevalonate metabolism: fact and artifact. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1023–1030. doi: 10.1016/0006-291x(77)91107-x. [DOI] [PubMed] [Google Scholar]


