Abstract
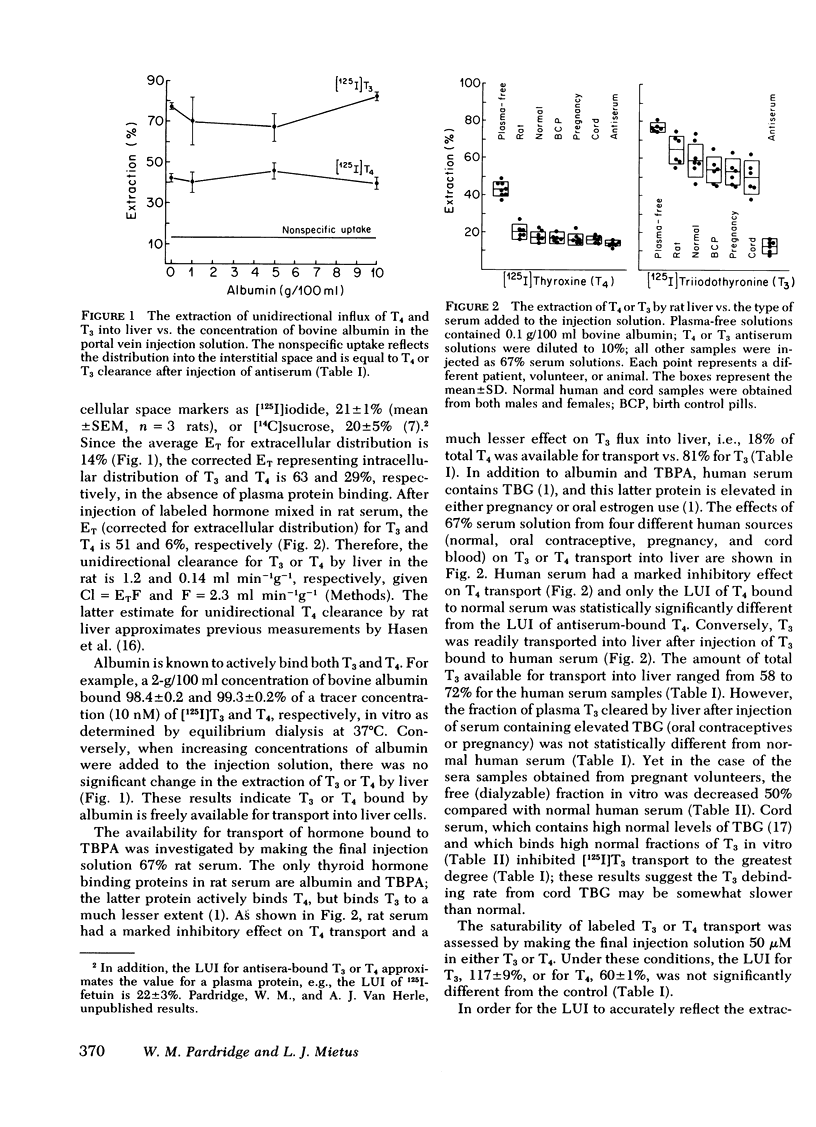
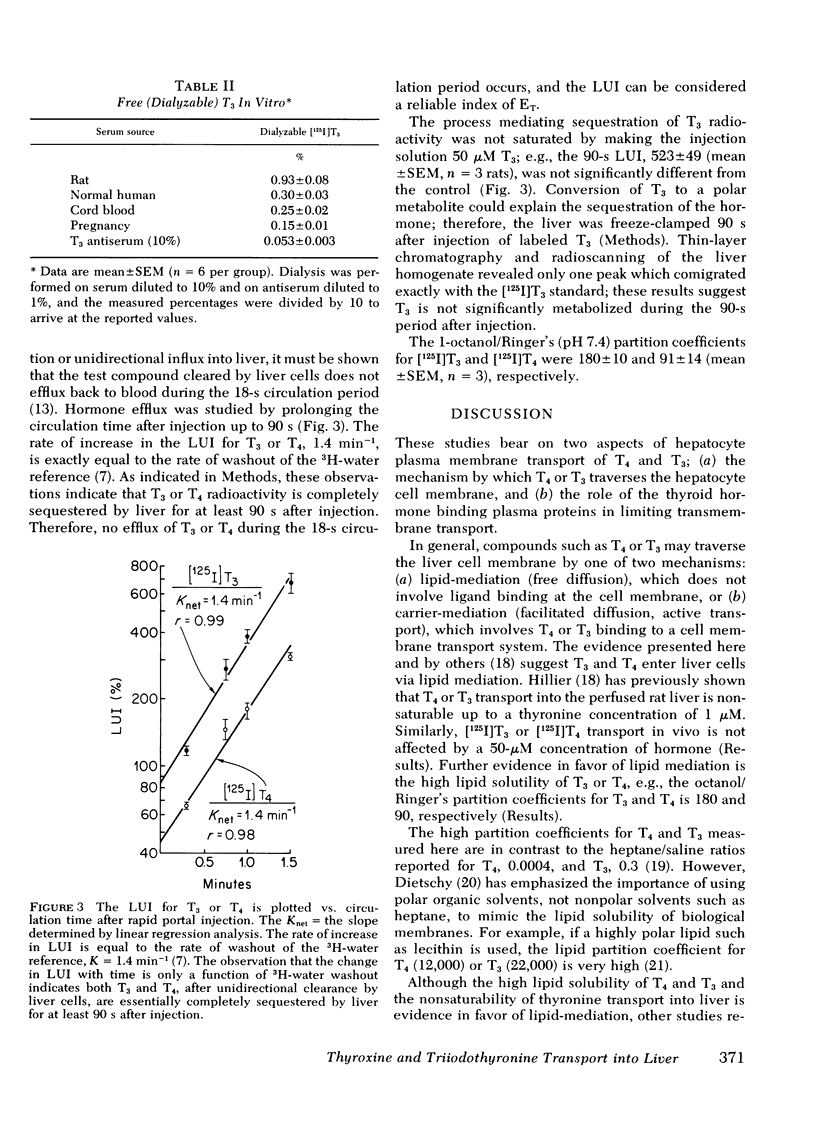
The transport of [125I]thyroxine (T4) and [125I]triiodothyronine (T3) into liver was investigated with a tissue sampling-portal vein injection technique in the anesthetized rat. The method allows the investigation of the effects of plasma proteins in human serum on the unidirectional influx of T4 or T3 into liver cells. The percent extraction of unidirectional clearance of T3 and T4 was 77±2% and 43±2%, respectively, after portal injection of a bolus of Ringer's solution. Cell membrane transport of T4 or T3 was nonsaturable because 50-μM concentrations of unlabeled hormone had no effect on transport. The addition of bovine albumin in concentrations of 1, 5, or 10 g/100 ml bound >98% of T4 or T3 in vitro, but had no significant effect on T3 or T4 transport in vivo. Conversely, 10% rabbit antisera specific for T3 or T4, completely abolished the intracellular distribution of thyroid hormone into liver. In the presence of rat serum, which contains albumin and thyroid hormone binding pre-albumin (TBPA), 18 and 81% of total plasma T4 and T3, respectively, were available for transport in vivo. The fraction of hormone available for transport in the presence of normal human serum, which contains albumin, TBPA, and thyroid hormone binding globulin (TBG) was 11% for T4 and 72% for T3. The fraction of hormone transported into liver after injection of serum obtained from pregnant or birth control pilltreated volunteers was 4% for T4 (but this was not significantly different from zero) and 54% for T3.
These data suggest: (a) The mechanism by which T4 and T3 traverse the liver cell membrane is probably free diffusion. (b) Albumin-bound T4 or T3 is freely cleared by liver, ∼50% of TBG-bound T3 is transported, but little, if any, of TBPA-bound T4 or TBG-bound T4 is cleared by liver cells. (c) Although the albumin-bound fraction of T4 greatly exceeds the free (dialyzable) moiety, the two fractions are both inversely related to the existing TBA or TBG level; therefore, in vitro measurements of free T4 would be expected to accurately reflect what is available for transport in vivo. Conversely, TBG-bound T3 is readily transported in vivo; therefore, it is proposed that in vitro measurements of free T3 do not reliably predict the fraction of T3 available for transport into liver in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burman K. D., Read J., Dimond R. C., Strum D., Wright F. D., Patow W., Earll J. M., Wartofsky L. Measurement of 3,3',5'-Triiodothyroinine (reverse T3), 3,3'-L-diiodothyronine, T3 and T4 in human amniotic fluid and in cord and maternal serum. J Clin Endocrinol Metab. 1976 Dec;43(6):1351–1359. doi: 10.1210/jcem-43-6-1351. [DOI] [PubMed] [Google Scholar]
- Dixon P. F. The kinetics of the exchange between transcortin-bound and unbound cortisol in plasma. J Endocrinol. 1968 Apr;40(4):457–465. doi: 10.1677/joe.0.0400457. [DOI] [PubMed] [Google Scholar]
- Dowling J. T., Appleton W. G., Musa B. U. Direct measurement of hepatic thyroxine flux in normal man. J Clin Endocrinol Metab. 1968 Oct;28(10):1503–1507. doi: 10.1210/jcem-28-10-1503. [DOI] [PubMed] [Google Scholar]
- Eckel J., Rao G. S., Rao M. L., Breuer H. Uptake of L-tri-iodothyronine by isolated rat liver cells. A process partially inhibited by metabolic inhibitors; attempts to distinguish between uptake and binding to intracellular proteins. Biochem J. 1979 Aug 15;182(2):473–491. doi: 10.1042/bj1820473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky C. A., Rose C. P. Blood-tissue exchange in liver and heart: the influence of heterogeneity of capillary transit times. Fed Proc. 1977 Nov;36(12):2629–2634. [PubMed] [Google Scholar]
- Hagen G. A., Elliott W. J. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973 Sep;37(3):415–422. doi: 10.1210/jcem-37-3-415. [DOI] [PubMed] [Google Scholar]
- Hagen G. A., Solberg L. A., Jr Brain and cerebrospinal fluid permeability to intravenous thyroid hormones. Endocrinology. 1974 Nov;95(5):1398–1410. doi: 10.1210/endo-95-5-1398. [DOI] [PubMed] [Google Scholar]
- Hamada S., Nakagawa T., Mori T., Torizuka K. Re-evaluation of thyroxine binding and free thyroxine in human serum by paper electrophoresis and equilibrium dialysis, and a new free thyroxine index. J Clin Endocrinol Metab. 1970 Aug;31(2):166–179. doi: 10.1210/jcem-31-2-166. [DOI] [PubMed] [Google Scholar]
- Hasen J., Bernstein G., Volpert E., Oppenheimer J. H. Analysis of the rapid interchange of thyroxine between plasma and liver and plasma and kidney in the intact rat. Endocrinology. 1968 Jan;82(1):37–46. doi: 10.1210/endo-82-1-37. [DOI] [PubMed] [Google Scholar]
- Heyns W., De Moor P. Kinetics of dissociation of 17-beta-hydroxysteroids from the steroid binding beta-globulin of human plasma. J Clin Endocrinol Metab. 1971 Feb;32(2):147–154. doi: 10.1210/jcem-32-2-147. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. Human thyroxine-binding globulin and thyroxine-binding pre-albumin: dissociation rates. J Physiol. 1971 Sep;217(3):625–634. doi: 10.1113/jphysiol.1971.sp009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A. P. The binding of thyroid hormones to phospholipid membranes. J Physiol. 1970 Dec;211(3):585–597. doi: 10.1113/jphysiol.1970.sp009295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A. P. The rate of triiodothyronine dissociation from binding sites in human plasma. Acta Endocrinol (Copenh) 1975 Sep;80(1):49–57. doi: 10.1530/acta.0.0800049. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. The release of thyroxine from serum protein in the vessels of the liver. J Physiol. 1969 Aug;203(2):419–434. doi: 10.1113/jphysiol.1969.sp008872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning E. P., Docter R., Bernard H. F., Visser T. J., Hennemann G. Active transport of triiodothyronine (T3) into isolated rat liver cells. FEBS Lett. 1978 Jul 1;91(1):113–116. doi: 10.1016/0014-5793(78)80029-5. [DOI] [PubMed] [Google Scholar]
- Musa B. U., Kumar R. S., Dowling J. T. Role of thyroxine-binding globulin in the early distribution of thyroxine and triiodothyronine. J Clin Endocrinol Metab. 1969 May;29(5):667–674. doi: 10.1210/jcem-29-5-667. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970 Dec 1;24(2):372–376. doi: 10.1016/0006-8993(70)90123-x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Hasen J. Estimation of rapidly exchangeable cellular thyroxine from the plasma disappearance curves of simultaneously administered thyroxine-131-I and albumin-125-I. J Clin Invest. 1967 May;46(5):762–777. doi: 10.1172/JCI105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Carrier-mediated transport of thyroid hormones through the rat blood-brain barrier: primary role of albumin-bound hormone. Endocrinology. 1979 Sep;105(3):605–612. doi: 10.1210/endo-105-3-605. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Jefferson L. S. Liver uptake of amino acids and carbohydrates during a single circulatory passage. Am J Physiol. 1975 Apr;228(4):1155–1161. doi: 10.1152/ajplegacy.1975.228.4.1155. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Effects of progesterone-binding globulin versus a progesterone antiserum on steroid hormone transport through the blood-brain barrier. Endocrinology. 1980 Apr;106(4):1137–1141. doi: 10.1210/endo-106-4-1137. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of protein-bound steroid hormones into liver in vivo. Am J Physiol. 1979 Oct;237(4):E367–E372. doi: 10.1152/ajpendo.1979.237.4.E367. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979 Jul;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Moeller T. L., Mietus L. J., Oldendorf W. H. Blood-brain barrier transport and brain sequestration of steroid hormones. Am J Physiol. 1980 Jul;239(1):E96–102. doi: 10.1152/ajpendo.1980.239.1.E96. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Tryptophan transport through the blood-brain barrier: in vivo measurement of free and albumin-bound amino acid. Life Sci. 1979 Oct 22;25(17):1519–1528. doi: 10.1016/0024-3205(79)90378-3. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Unidirectional influx of glutamine and other neutral amino acids into liver of fed and fasted rat in vivo. Am J Physiol. 1977 May;232(5):E492–E496. doi: 10.1152/ajpendo.1977.232.5.E492. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Ostberg L., Andersson L., Kamwendo F., Pertoft H. Studies on the transport and cellular distribution of vitamin A in normal and vitamin A-deficient rats with special reference to the vitamin A-binding plasma protein. J Biol Chem. 1973 Jun 10;248(11):4009–4022. [PubMed] [Google Scholar]
- Rao M. L., Rao G. S., Breuer H. Uptake of estrone, estradiol-17beta and testosterone by isolated rat liver cells. Biochem Biophys Res Commun. 1977 Jul 25;77(2):566–573. doi: 10.1016/s0006-291x(77)80016-8. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Brandon M. R. The thyroxine-binding properties of rat and rabbit serum proteins. Endocrinology. 1976 Jan;98(1):91–98. doi: 10.1210/endo-98-1-91. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Hecker E., Ingbar S. H. The effects of an acute load of thyroxine on the transport and peripheral metabolism of triiodothyronine in man. J Clin Invest. 1970 Apr;49(4):650–654. doi: 10.1172/JCI106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninovich A. A., Farach H., Ezrin C., Volpé R. Lack of significant binding of L-triiodothyronine by thyroxine-binding globulin in vivo as demonstrated by acute disappearance of 131-I-labeled triiodothyronine. J Clin Invest. 1966 Aug;45(8):1290–1301. doi: 10.1172/JCI105436. [DOI] [PMC free article] [PubMed] [Google Scholar]


