Abstract
Mechanical forces associated with blood flow play an important role in regulating vascular signaling and gene expression in endothelial cells (ECs). MicroRNAs (miRNAs) are a class of noncoding RNAs that posttranscriptionally regulate the expression of genes involved in diverse cell functions, including differentiation, growth, proliferation, and apoptosis. miRNAs are known to have an important role in modulating EC biology, but their expression and functions in cells subjected to shear stress conditions are unknown. We sought to determine the miRNA expression profile in human ECs subjected to unidirectional shear stress and define the role of miR-21 in shear stress-induced changes in EC function. TLDA array and qRT-PCR analysis performed on HUVECs exposed to prolonged unidirectional shear stress (USS, 24 hrs, 15 dynes/cm2) identified 13 miRNAs whose expression was significantly upregulated (p < 0.05). The miRNA with the greatest change was miR-21; it was increased 5.2-fold (p = 0.002) in USStreated versus control cells. Western analysis demonstrated that PTEN, a known target of miR-21, was downregulated in HUVECs exposed to USS or transfected with pre-miR-21. Importantly, HUVECs overexpressing miR-21 had decreased apoptosis and increased eNOS phosphorylation and nitric oxide (NO) production. These data demonstrate that shear stress forces regulate the expression of miRNAs in ECs, and that miR-21 influences endothelial biology by decreasing apoptosis and activating the NO pathway. These studies advance our understanding of the mechanisms by which shear stress forces modulate vascular homeostasis.
Keywords: microRNA, shear stress, endothelium, apoptosis, nitric oxide synthase
Introduction
Mechanical forces created by pulsatile blood pressure and flow play an essential role in modulating circulatory function and vascular homeostasis. The primary sensors of mechanical forces in the vessel wall are endothelial cells (ECs), and mechanical forces can induce dramatic changes in EC signaling and gene expression that in turn modulate cell morphology, migration, growth, proliferation, apoptosis, and the production of vasoactive substances[1]. ECs are primarily subjected to shear stress, the force that acts parallel to the luminal surface of the vessel and is a product of fluid viscosity and the velocity gradient between adjacent layers of flowing fluid.
miRNAs have become a major focus of molecular biology research because they posttranscriptionally regulate the expression of genes involved in important cellular processes, including differentiation, growth, and apoptosis. Recently, the miRNA expression profiles in quiescent human ECs was described[2; 3; 4], but relatively few studies have described the role of specific miRNAs in EC function in response to physiologic stimuli. Here, we report that unidirectional shear stress (USS) enhanced the expression of a distinct group of miRNAs, including miR-21. We found that expression of miR-21 in HUVECs had an impact on apoptosis and the PI3K/Akt/eNOS pathway. These data provide insight into the role of miRNAs in shear stress-induced changes in EC gene expression and phenotype.
Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs, Genlantis), passages 2-6, were exposed to USS by using a cone-in-plate viscometer with a 1 ° angle to create 15 dynes/cm2 for 24 hours[5].
HUVEC transfections were generally performed 24 hours after splitting (see online supplement). Additional treatment included LPS (Lipopolysaccharide, Sigma) or LY294002 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, Biosource). LY294002 was dissolved in DMSO (dimethylsulfoxide, Sigma) and used in a 10 μM concentration. LPS was diluted in water and used in a 10 ng/μl concentration in medium containing 0.5% fetal calf serum.
TaqMan Low Density Array (TLDA)
Total RNA from USS or nonsheared HUVECs was isolated using the mirVana kit (Applied Biosystems). The Human MicroRNA v2 TLDA miRNA array was performed by the Emory Biomarker Service Center. miRNA Ct values were normalized to control RNU48 and converted into copy numbers, where the copy number = 2(−Ct).
microRNA qRT-PCR
Quantitative assessment of specific miRNA levels was measured using standard protocols from Applied Biosystems and Qiagen (see online supplement).
Caspase 3 activity
For assessment of caspase-3 activity, HUVECs were treated with 10 ng/ml LPS in 0.5% FBS medium for 24 hours. Caspase-3 activity was measured with the ApoAlert Caspase 3 Colorimetric Assay Kit (Clontech) according to the manufacturer's instructions (see online supplement).
Western Analysis
Western analysis was performed as previously described[6]. Antibodies against p-Akt (Ser473), total Akt, p-eNOS (Ser1177, Ser113, Thr495) and Dicer were obtained from Cell Signaling; these were used in a 1:1000 dilution. Anti-total eNOS (BD Transduction Laboratories) was diluted 1:2500. Anti-PTEN (1:500) was purchased from Abcam. Control antibodies were anti-α-actin (Sigma, 1:1000) and anti-GAPDH (Santa Cruz, 1:1000).
NO measurements
Measurement of NO was assessed by electron spin resonance (ESR) as described earlier[7] (see online supplement).
Results
USS upregulates miRNA expression
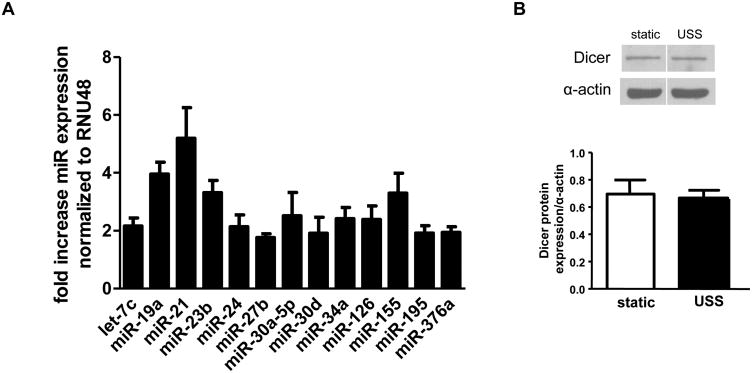
In a qRT-PCR-based screen of 384 miRNAs in HUVECs subjected to prolonged USS (24 hours, 15 dynes/cm2) or static conditions, 13 miRNAs were significantly upregulated (Figure 1A). None of the screened miRNAs were significantly downregulated by USS (online supplement). miR-21 was the most highly upregulated; its expression was increased 5.2-fold by USS. Other upregulated miRNAs included miR-155, miR-19a, miR-34a, miR-126, miR-24, miR-27b, members of the miR-30 family, and let-7c. Several of these miRNAs have previously been shown to be highly expressed in ECs[2; 4; 8]. Importantly, USS does not alter expression of Dicer, the endonuclease responsible for processing pre-miRNA (Figure 1B), indicating that miRNA upregulation was not due to changes in miRNA processing by Dicer. Regulation of Dicer expression has been suggested as one mechanism by which miRNAs modulate EC gene expression[9].
Figure 1.
A: USS significantly upregulated miRNAs in HUVECs. p<0.05, fold change USS versus nonsheared cells (n = 3-25). B: Western analysis (different parts of same blot) of Dicer expression in HUVECs under static and USS conditions.
miR-21 overexpression modulates apoptosis in HUVECS
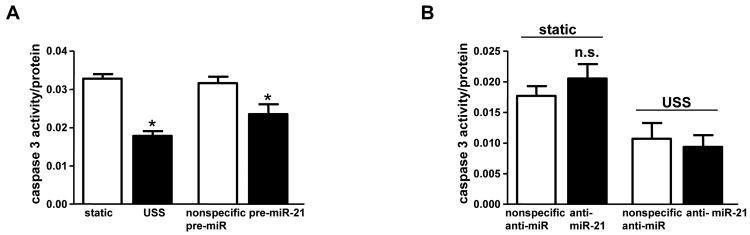
We focused on miR-21 because it was the most upregulated by USS. USS is known to attenuate EC apoptosis[10]; we found that USS decreased caspase-3 activity 45.6% (Figure 2A). We hypothesized that miR-21 was involved in modulating the decrease in EC apoptosis by USS because of its role in modulating apoptosis in different adult tissues[11]. To test this hypothesis, HUVECs were transfected with miR-21 precursor (pre-miR-21), which increased miR-21 levels (online supplement). In the absence of USS, miR-21 overexpression attenuated caspase-3 activity by 25.7% (Figure 2A).
Figure 2.
Apoptosis in HUVECs subjected to USS, pre-, or anti-miR-21 transfection. A: caspase-3 activity in cells subjected to USS (* p=0.0001 vs static, n=4) or pre-miR-21 transfection (*p=0.017 vs control transfection, n=9). B: caspase-3 activity in cells transfected with anti-miR-21, with and without USS (n=6-10).
Transfection of anti-miR-21 (antisense miRNA that reduced miR-21 levels by 65%, online supplement) into static or sheared HUVECs did not alter caspase-3 activity significantly (Figure 2B). miR-21 levels are relatively low in static HUVECs, so we did not expect to see an effect of anti-miR-21 transfection in these cells. In HUVECs subjected to USS, caspase-3 activity was relatively low, so we may have been unable to detect modest changes in caspase-3 activity in response to anti-miR-21 transfection. Together, the miR-21 overexpression and inhibition data suggest that miR-21 contributes to the antiapoptotic effect of USS, but miR-21 expression alone is not completely necessary for USS-induced changes in EC apoptosis.
USS and miR-21 overexpression attenuate PTEN expression
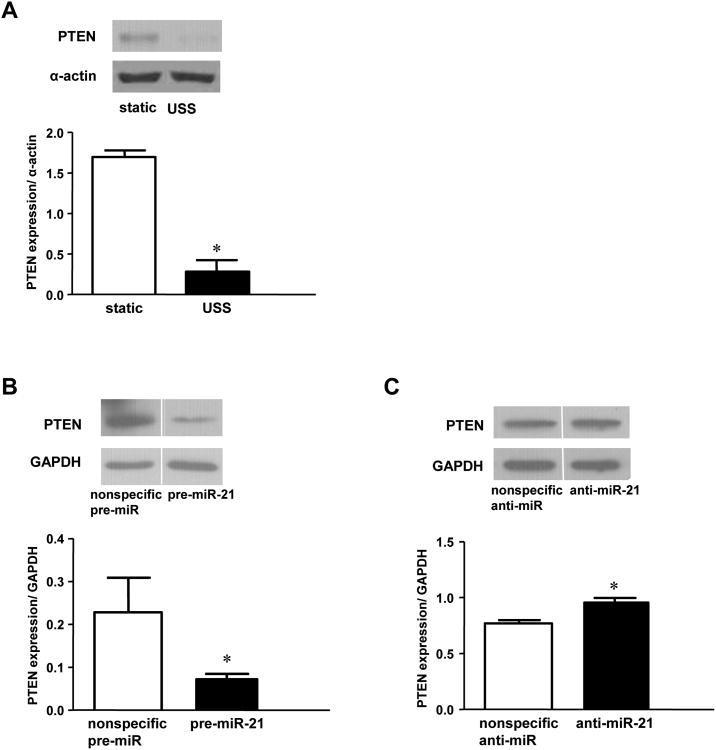
miR-21 has been shown to posttranscriptionally regulate the expression of the proapoptotic gene PTEN[12]. Recently, miR-21 was demonstrated to attenuate apoptosis of vascular smooth muscle cells by downregulation of PTEN[12]. Overexpression of miR-21 in static HUVECs reduced PTEN protein expression by 68% (Figure 3B). In comparison, USS decreased PTEN levels by 83% compared to controls (Figure 3A). Furthermore, PTEN expression was modestly increased in sheared HUVECs after anti-miR-21 transfection (Figure 3C). Together, these results are consistent with negative regulation of PTEN by miR-21 in HUVECs. Importantly, anti-miR-21 had no effect on PTEN expression in nonsheared ECs (data not shown), indicating that the impact of miR-21 on PTEN expression was most apparent when levels of miR-21 were increased by USS or pre-miR-21 transfection.
Figure 3.
PTEN protein expression in HUVECs after USS, pre-, or anti-miR-21 transfection. A: Western analysis and densitometry of USS versus static conditions (*p=0.0009, n=3). B: Western analysis (different parts of same blot) and densitometry of static HUVECs transfected with pre-miR-21 or control (*p=0.0159, n=5). C: Western analysis (different parts of same blot) and densitometry of cells transfected with anti-miR-21 or nonspecific anti-miRNA and sheared for 24 hours (15 dynes/cm2). *p = 0.0028 (n = 7).
miR-21 overexpression enhances Akt phosphorylation, eNOS phosphorylation and NO production
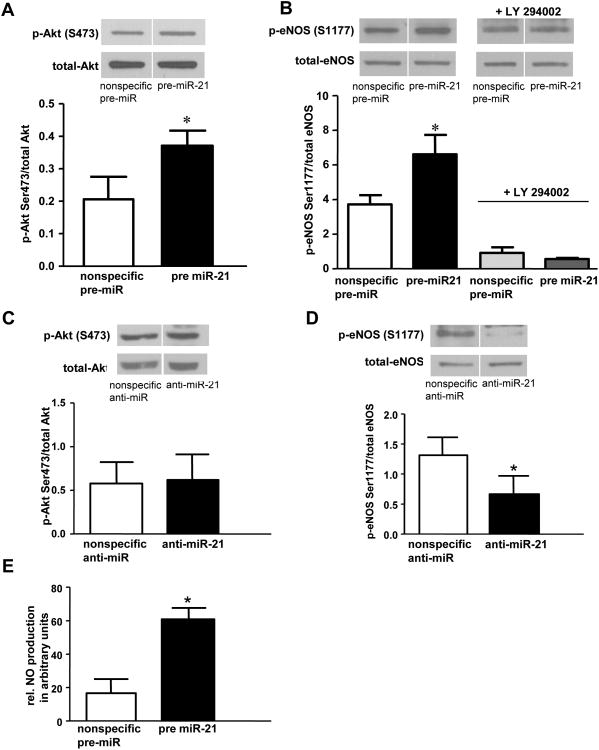
PTEN is a lipid phosphatase that dephosphorylates phosphatidylinositol 3,4,5-triphosphate and thereby antagonizes PI3K and the PI3K/Akt survival pathway. The PI3K/Akt pathway is involved in decreasing apoptosis in various cells types [12],[13]. Further, Akt activates eNOS in ECs by phosphorylation of eNOS at serine 1177, resulting in increased activity of the enzyme and enhanced NO. production. We examined the impact of miR-21 overexpression in HUVECs on Akt activation and found an increase in p-Akt (serine 473, Figure 4A) compared to controls. miR-21 overexpression also increased phosphorylation of eNOS at serine 1177 (Figure 4B), an effect that was inhibited by LY 294002, a PI3K inhibitor (Figure 4B). Total eNOS levels were not affected by miR-21 overexpression, nor was phosphorylation of eNOS at other well-known sites (i.e. serine 633, serine 113, and threonine 495, not shown).
Figure 4.
A-D: Phospho-Akt and phospho-eNOS protein in HUVECs transfected with pre, or anti-miR-21. A: Western analysis (different parts of same blot) and densitometry of p-Akt (serine 473) after transfection with pre-miR-21 for 28 hours; *p=0.03 vs control transfection (n=3); B: Western (different parts of same blot) and densitometry of p-eNOS (serine 1177) after transfection with pre-miR-21 or control pre-miRNA for 48 hours, +/- LY 294002 (10 mM); *p=0.04 (n=6). C: Western analysis (different parts of same blot) and densitometry of p-Akt in cells transfected with anti-miR-21 or control, then subjected to USS (15 dynes/cm2) for 24 hours. D: p-eNOS expression (different parts of same blot) in cells transfected with anti-miR-21 or control, then subjected to USS (15 dynes/cm2) for 24 hours. *p=0.037 (n=3). E: NO production (expressed as ESR arbitrary units) in HUVECs transfected with pre-miR-21 or control, *p=0.007 (n=4).
Interestingly, anti-miR-21 transfection had no effect on Akt phosphorylation in HUVECs (Figure 4C), but anti-miR-21 transfection significantly attenuated eNOS phosphorylation (Figure 4D). These latter findings in regard to Akt phosphorylation are likely related to the transient nature of Akt activation in response to prolonged USS. Akt phosphorylation is maximally enhanced after one hour of USS and gradually returns to baseline levels after 12 to 18 hours of shear stress[10]. Because our analysis was performed after 24 hours of USS, we would have missed an effect of anti-miR-21 on the transient increase in Akt phosphorylation. When the results of the pre-miR-21 and anti-miR-21 transfection experiments are considered together, we believe that miR-21 enhances activity of the PI3K/Akt/eNOS pathway in ECs through suppression of PTEN, a negative regulator of the PI3K/Akt/eNOS pathway[14].
NO is established as an important antiapoptotic factor; mechanisms that increase NO production or enhance NO bioavailability (including USS) protect against EC apoptosis[15]. Since miR-21 overexpression decreased EC apoptosis and increased eNOS phosphorylation, we assessed whether these changes were associated with enhanced NO. bioavailability. HUVECs overexpressing miR-21 had a 3.7-fold increase in NO. production compared to cells transfected with a nonspecific pre-miRNA (Figure 4E). This very novel finding shows that miR-21 expression can modulate NO availability and supports our hypothesis that miR-21 expression plays a role in USS-induced changes in EC apoptosis through enhancement of the PI3K/Akt/eNOS signaling pathway.
Discussion
The main findings of this study are that USS, an important regulator of vascular homeostasis, induced expression of a distinct group of miRNAs in human ECs, and that expression of one of these miRNAs, miR-21, modulated EC apoptosis and NO. production. Our data suggest that the effects of miR-21 expression on ECs involve regulation of PTEN expression and subsequent changes in the PI3K/Akt/eNOS pathway.
In the arterial tree, regional differences in shear stress forces produce distinct effects on the EC phenotype. USS, present in the straight portions of the tree, elicits a potent anti-inflammatory and atheroprotective response in ECs [1]. In this study, we focused on this atheroprotective shear stress force and found an upregulation of a distinct group of miRNAs. We hypothesized that these miRNAs may be involved in modulating the atheroprotective effects of USS, including attenuation of EC apoptosis. In contrast, oscillatory shear stress, which is the shear stress force present at branch points and curved regions of arterial tree, is known to induce a pro-inflammatory and atherosclerotic response in ECs. We did not study oscillatory shear stress here, but we suspect that this stimulus induces its own distinct miRNA expression profile.
miR-21 is an important regulator of vascular smooth muscle apoptosis [12] and is known to be regulated in human cancers [11] and cardiac hypertrophy[16]. We found that overexpression of miR-21 in HUVECs attenuated apoptosis, although this effect was more modest than that observed with USS. This suggests that changes in EC expression induced by miR-21 are just one part of the mechanism responsible for USS-induced changes in EC apoptosis. This concept is supported by the minimal effect of anti-miR-21 on EC apoptosis; however, this latter finding may also be due to our inability to completely inhibit miR-21 with the anti-miRNA transfection technique. In general, miRNA levels in ECs were relatively low, so further inhibition by anti-miRNA may not have had a dramatic impact. In addition, USS upregulated a group of miRNAs, several of which have been implicated in regulation of the apoptotic pathway. Therefore, by focusing on one miRNA, we may have not appreciated the full impact of USS-induced changes in miRNA expression on EC apoptosis.
Our data indicate that one of the targets for miR-21 in ECs is PTEN. PTEN is expressed at a relatively high level in all adult tissues [17], including ECs, and PTEN has been shown to play an important role in regulating apoptosis through antagonism of the PI3K/Akt pathway[11]. In addition, PTEN has been shown to negatively regulate eNOS phosphorylation and NO. production through antagonism of the PI3K/Akt pathway[14]. Here, we describe a novel and important link between miR-21, PTEN expression and NO production, demonstrating that simple overexpression of miR-21 suppressed PTEN expression as well as increased Akt phosphorylation, eNOS phosphorylation and NO. production in human ECs. Although we believe this link is important, we must consider that miR-21 targets other mRNAs in ECs that could also be involved in modulating EC apoptosis. PDCD4 (Programmed cell death 4) is another proapoptotic gene that is a known target of miR-21[18], but we were unable to observe a significant change in its expression in HUVECs after pre-miR-21 transfection (not shown). We are currently assessing other potential targets of miR-21 in human ECs.
Conclusion
We conclude that upregulation of miR-21 in response to prolonged USS contributes to the ability of USS to increase NO bioavailability and reduce EC apoptosis. In this study, we demonstrated that shear stress forces upregulated multiple miRNAs in ECs, but we showed that EC biology could be altered by overexpression of a single miRNA. It is likely that the myriad of changes in EC phenotype induced by USS involve the concerted action of multiple miRNAs, and future work will need to examine the role of other shear-regulated miRNAs in EC biology, both individually and as a group.
Supplementary Material
Acknowledgments
We thank Drs. Sergey Dikalov and Alfiya Bikineyeva for assistance with ESR measurements.
Sources of Funding: NIH grants R01HL077274 and HL80711. The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- EC
endothelial cell
- ESR
electron spin resonance
- HUVEC
human umbilical vein endothelial cell
- miRNA
microRNA
- PI3K
phosphatidylinositol 3′-kinase
- PTEN
Phosphatase and TENsin homolog or phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase
- qRT-PCR
quantitative real-time polymerase chain reaction
- USS
unidirectional shear stress
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–24. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 2.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 3.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–71. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 4.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 5.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–85. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 6.Ramasamy S, Parthasarathy S, H DG. Regulation of endothelial nitric oxide synthase gene expression by oxidized linoleic acid. J Lipid Res. 1998;39:268–276. [PubMed] [Google Scholar]
- 7.Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 8.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–7. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asada S, Takahashi T, Isodono K, Adachi A, Imoto H, Ogata T, Ueyama T, Matsubara H, Oh H. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2512–21. doi: 10.1152/ajpheart.00233.2008. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–41. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Lee CG. MicroRNA and cancer--focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760–6. doi: 10.1074/jbc.M110219200. [DOI] [PubMed] [Google Scholar]
- 14.Church JE, Qian J, Kumar S, Black SM, Venema RC, Papapetropoulos A, Fulton DJ. Inhibition of endothelial nitric oxide synthase by the lipid phosphatase PTEN. Vascul Pharmacol. 2009 doi: 10.1016/j.vph.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–64. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–41. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of MicroRNAs in Hydrogen Peroxide-mediated Gene Regulation and Cellular Injury Response in Vascular Smooth Muscle Cells. J Biol Chem. 2009;284:7903–13. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.