Summary
Here, we demonstrate that the fractalkine(FKN)/CX3CR1 system represents a previously undescribed regulatory mechanism for pancreatic islet beta cell function and insulin secretion. CX3CR1 KO mice exhibited a marked defect in glucose and GLP1-stimulated insulin secretion, and this defect was also observed in vitro in isolated islets from CX3CR1 KO mice. In vivo administration of FKN improved glucose tolerance with an increase in insulin secretion. In vitro treatment of islets with FKN increased intracellular Ca2+ and potentiated insulin secretion in both mouse and human islets. The KO islets exhibited reduced expression of a set of genes necessary for the fully functional, differentiated beta cell state, whereas, treatment of WT islets with FKN led to increased expression of these genes. Lastly, expression of FKN in islets was decreased by aging and HFD/obesity, suggesting that decreased fractalkine/CX3CR1 signaling could be a mechanism underlying beta cell dysfunction in type 2 diabetes.
Keywords: Fractalkine, CX3CR1, beta cell, insulin secretion, diabetes
Introduction
The prevalence of Type 2 diabetes has risen dramatically in the United States and globally and has now reached epidemic proportions (Olefsky and Glass, 2010). The etiology of this disease involves both insulin resistance and decreased beta cell insulin secretion, and one typically needs both defects (two hit hypothesis) in order to develop the hyperglycemic diabetic state (Defronzo, 2009; Olefsky and Glass, 2010; Weir and Bonner-Weir, 2004). Beta cell failure in type 2 diabetes is associated with at least 2 major mechanisms: reduced overall beta cell mass and decreased insulin secretory function per beta cell (Weir and Bonner-Weir, 2004). In the prediabetic, insulin resistant state, islets respond to the increased insulin demand with enhanced insulin secretion and increased beta cell mass to generate compensatory hyperinsulinemia and maintain relative euglycemia. However, when type 2 diabetes emerges, beta cell function and mass are significantly decreased, with insufficient insulin secretion to compensate for the insulin resistance, resulting in the chronic hyperglycemic diabetic state. This beta cell dysfunction is largely manifested as impaired glucose-stimulated insulin secretion (GSIS) and can be detected in the earliest stages of type 2 diabetes with complete loss of first phase GSIS (Defronzo, 2009; Weir and Bonner-Weir, 2004). On the other hand, decreased beta cell mass is usually not present at the time of diagnosis of Type 2 Diabetes (Rahier et al., 2008), suggesting that loss of beta cell mass is not responsible for the onset of type 2 diabetes, but rather is a consequence of diabetes.
Recently, it has been proposed that beta cell dysfunction in diabetes is associated with progressive dedifferentiation of beta cells (Jonas et al., 1999; Weir and Bonner-Weir, 2004). This is accompanied by reduced expression of genes necessary for maintaining the mature beta cell phenotype, including PDX-1, Glut2 and insulin, with increased expression of proliferative genes such as c-myc (Jonas et al., 1999; Rahier et al., 2008). This may provide a mechanism for increasing beta cell mass, at the expense of decreased beta cell function.
Fractalkine (also known as CX3CL1 or neurotactin; FKN) is a CX3C chemokine, and is expressed in neurons, endothelial cells, hepatocytes and vascular smooth muscle cells (Aoyama et al., 2010; Cardona et al., 2006; Haskell et al., 1999; Lucas et al., 2001; Zernecke et al., 2008). FKN is produced as a membrane-bound protein, and mediates cell-to-cell adhesion and communication by binding to its cognate receptor CX3CR1 (also known as GPR13) (Combadiere et al., 2003; Imai et al., 1997; Lesnik et al., 2003; Tacke et al., 2007; Teupser et al., 2004; Zernecke et al., 2008). For example, membrane-bound FKN promotes cell:cell adhesion, and plays a role in the attachment of monocytes/macrophages to CX3CR1 expressing cell types (Haskell et al., 1999; Zernecke et al., 2008). In liver, FKN expressed in hepatocyte and stellate cells is anti-fibrotic and can suppress inflammatory activation of Kupffer cells (Aoyama et al., 2010). In the brain, FKN mediates interactions between neurons and glial cells (Cardona et al., 2006). A soluble form of FKN is generated through proteolytic cleavage at the base of the mucin-like stalk, mediated by ADAM 10 and ADAM 17 (Garton et al., 2001; Hundhausen et al., 2003), producing an extracellular form of FKN which can regulate target cells by paracrine mechanisms. Furthermore, cleaved soluble FKN enters the circulation where it can have potential endocrine effects (Shah et al., 2011).
In this study, we have discovered a regulatory pathway for the FKN/CX3CR1 system in the modulation of beta cell insulin secretory function. We found that CX3CR1 KO mice develop hyperglycemia with reduced nutrient-stimulated insulin secretion and that isolated islets from KO mice produce less insulin in response to a variety of stimuli compared to WT islets. Furthermore, in vivo FKN administration leads to increased plasma insulin levels with improved glucose tolerance, while in vitro FKN treatment of isolated islets directly enhances beta cell insulin secretion.
Results
CX3CR1-deficient mice exhibit impaired glucose tolerance with reduced insulin secretion
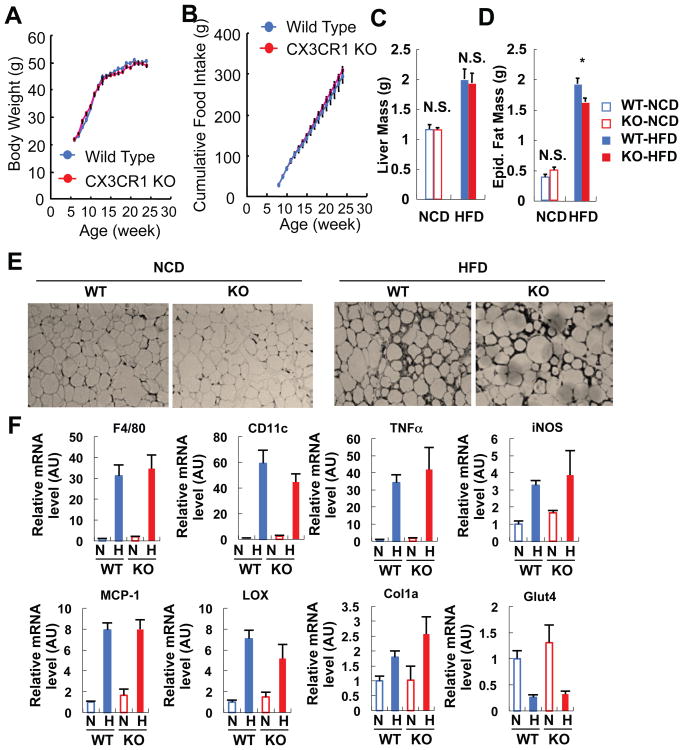
Obesity causes inflammation and insulin resistance and the FKN/CX3CR1 system plays a role in monocyte attachment and immune cell migration (Combadiere et al., 2003; Hotamisligil et al., 1995; Lee et al., 2010; Lee et al., 2011; Lesnik et al., 2003; Olefsky and Glass, 2010; Tacke et al., 2007; Teupser et al., 2004). To address the potential effect of this receptor on obesity-induced inflammation, we studied lean and obese CX3CR1 KO mice. The KO mice exhibited normal food intake, body weight gain, and liver mass either on chow or high fat diet (HFD) (Figures 1A-1C). Adipose tissue mass was the same between KO and WT mice on chow diets, but was slightly lower in the KO mice on HFD (Figure 1D). Interestingly, we found no evidence that FKN or CX3CR1 play a role in macrophage accumulation in adipose tissue or liver or in inflammation-induced insulin resistance. For example, macrophage infiltration (Figure 1E) and expression of macrophage marker genes, such as F4/80 and CD11c (Figure 1F), was not altered in the adipose tissue of KO mice. Moreover, CX3CR1 KO did not affect HFD-induction of genes involved in inflammation (iNOS, MCP-1, and TNF-α) or fibrosis (lysyl oxidase and collagen 1a) in adipose tissue (Figure 1F). Consistent with this, Lumeng also recently reported that CX3CR1 deficiency is without effect on adipose tissue macrophage content in HFD mice (Morris et al., 2012). Furthermore, the decrease in GLUT4 expression which typically occurs on HFD was not attenuated by the CX3CR1 KO (Figure 1F), suggesting that CX3CR1 KO does not affect insulin resistance.
Figure 1.
CX3CR1 KO mice exhibit normal body weight, food intake, fat and liver mass, and inflammatory and metabolic gene expression in adipose tissue. (A) Body weight change on HFD. Mean+/-SEM, n=20 for both WT and KO. (B) Cumulative food intake on HFD. Food intake was measured from 5 different cages per group, and 4 mice were housed in each cage. Mean+/-SEM. (C) Liver mass. Mean+/-SEM, n=8 per group. N.S., not significant. NCD, normal chow diet. (D) Epididymal fat mass. Mean+/-SEM, n=8 per group. *, P<0.05. (E) Immunohistochemistry analysis of epididymal adipose tissue using anti-F4/80 antibody. Representative figures were presented from the analyses of 5 different mice per group. (F) mRNA expression of inflammatory and metabolic genes in epididymal adipose tissue on NCD and HFD. Mean+/-SEM, n=5 per group. N, normal chow diet; H, high fat diet. AU, arbitrary unit.
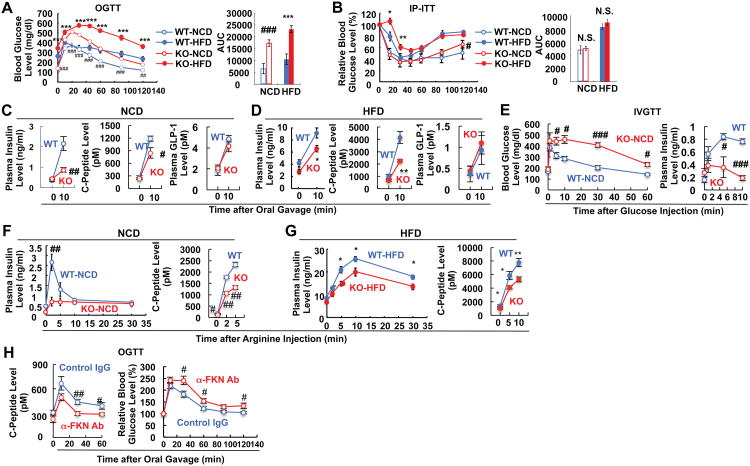
Unexpectedly, both lean/chow-fed and obese/HFD CX3CR1 KO mice developed glucose intolerance compared to wild type (WT) mice upon oral glucose administration, and this effect was exacerbated in the obese state (Figure 2A). Despite the glucose intolerance, these mice exhibited normal insulin sensitivity, as shown by insulin tolerance testing (Figure 2B), suggesting that a defect in insulin secretion was the cause of the hyperglycemia. To assess this, we measured circulating insulin and C-peptide levels during the oral glucose tolerance tests (OGTTs). Lean chow-fed and obese HFD KO mice displayed decreased insulin and C-peptide secretion with normal GLP1 levels (Figures 2C and 2D) (Figure S1), compared to their WT counter parts, indicating that CX3CR1 deficiency causes a beta cell insulin secretory defect. Interestingly, the glucose intolerance and the defect in insulin secretion in the CX3CR1 KO mice was more pronounced after an intravenous (IV) glucose challenge, and the differences between WT and KO mice were quantitatively greater than after oral glucose (Figure 2E). Furthermore, insulin secretion provoked by intraperitoneal (IP) arginine administration was reduced in both lean/chow-fed and obese/HFD CX3CR1 KO mice (Figures 2F and 2G), further indicating that CX3CR1 deficiency causes a beta cell insulin secretory defect.
Figure 2.
CX3CR1 KO mice exhibit impaired glucose tolerance due to reduced insulin secretion. (A-D) CX3CR1 KO mice manifest impaired glucose tolerance with normal insulin sensitivity either on NCD (n=11) or HFD (n=12). Mean+/-SEM. (A) Oral glucose tolerance test. ##, P<0.01 WT-NCD vs KO-NCD; ###, P<0.001 WT-NCD vs KO-NCD; ***, P<0.001 WT-HFD vs KO-HFD. AUC, area under the curve. (B) Insulin tolerance test. *, P<0.05 WT-HFD vs KO-HFD; **, P<0.01 WT-HFD vs KO-HFD; N.S., not significant. (C) Plasma insulin (left), C-Peptide (middle), and GLP1 (right) levels of NCD mice during OGTT in panel A. #, P<0.05; ##, P<0.01. (D) Plasma insulin, C-peptide, and GLP1 levels of HFD mice during OGTT in panel A. *, P<0.05; **, P<0.01. (E) Intravenous glucose tolerance test (left) and plasma insulin level during IVGTT (right). Mean+/-SEM, n=4 per group. (F-G) Plasma insulin and C-Peptide levels of NCD (F) or HFD (G) mice during arginine tolerance test. Mean+/-SEM, n=8 per group. #, P<0.05; ##, P<0.01; *, P<0.05; **, 0.01. (H) Fractalkine neutralization reduces insulin secretion and causes glucose intolerance in WT mice. FKN neutralizing antibody was injected IP to WT mice, and 30 min later the mice were given oral gavage of glucose (2g/kg) for GTT. Mean+/-SEM, n=10 per group. Left, plasma insulin level during GTT; right, GTT. See also Figure S1.
In complementary experiments, we injected anti-FKN antibodies into mice to neutralize circulating FKN. This led to decreased C-peptide levels and glucose intolerance (Figure 2H), fully consistent with the results in the KO mice, indicating that ongoing stimulation of CX3CR1 is required for normal insulin secretion and glycemic control.
CX3CR1 KO islets display reduced insulin secretion with decreased expression of genes associated with beta cell function
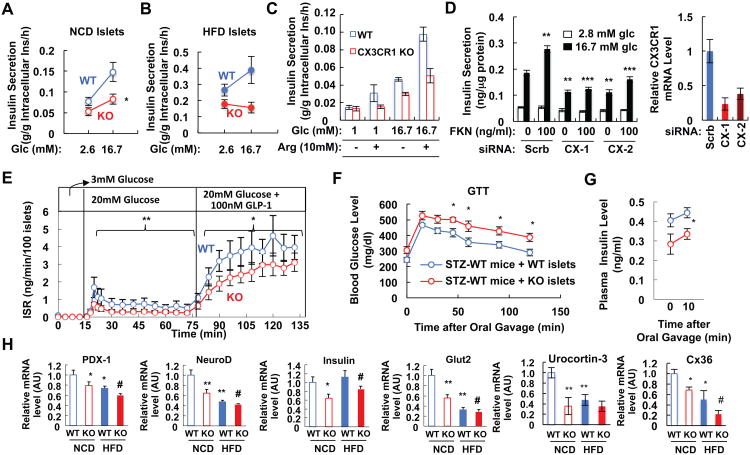
To directly test beta cell function, and to determine whether the in vivo insulin secretory defects are primary or secondary, we carried out in vitro systems on isolated islets and Min6 cells. First, to directly test the function of CX3CR1 in beta cells, we measured insulin secretion under static incubation conditions by isolated islets obtained from chow and HFD WT and CX3CR1 KO mice. As seen in Figure 3A, the KO islets exhibited a marked decrease in GSIS which was more profound in islets obtained from HFD KO mice (Figure 3B). Moreover, CX3CR1 KO islets exhibited reduced insulin secretion in response to arginine (Figure 3C), consistent with the in vivo results seen in Figure 2F. To demonstrate the cell autonomous effects of CX3CR1 on GSIS in another way, and to show that they are independent of in vivo developmental issues, we used RNAi interference to deplete CX3CR1 in Min6 cells in vitro (Figure 3D). Two different anti-CX3CR1 siRNAs led to CX3CR1 knockdown and both attenuated GSIS and abrogated FKN effects on insulin secretion. We subsequently measured time-dependent insulin secretion by WT and KO islets using perifusion analysis. The KO islets demonstrated significantly lower insulin secretion rates compared to WT in response to both high glucose and GLP1 stimulation (Figure 3E). Interestingly, oxygen consumption rates by WT and CX3CR1 KO islets were comparable (Figure S2), implying that CX3CR1 KO does not affect metabolic pathways or mitochondrial respiration. These in vitro experiments demonstrate that the effects of CX3CR1 deletion are cell autonomous and not secondary to other in vivo events. To test that the affect of CX3CR1 KO is intrinsic to the islet in vivo, we transplanted CX3CR1 KO and WT islets into the kidney capsule in streptozotocin (STZ)-induced diabetic mice. As seen in Figure 3F, transplantation of WT islets had a greater effect to lower glucose levels compared to transplantation of the KO islets. This was further confirmed by measurements of insulin secretion, which showed greater basal and insulin stimulated insulin levels in the STZ mice transplanted with WT islets compared to the STZ mice receiving the KO islets (Figure 3G). Thus, the insulin secretory defect in CX3CR1 KO islets is mediated by alterations in islet function independent of extra-islet mechanisms. Taken together, these data indicate that the beta cell FKN/CX3CR1 system is necessary for normal insulin secretory function in response to glucose, arginine, and GLP1, both in vitro and in vivo.
Figure 3.
CX3CR1 KO islets display reduced insulin secretion with lower expression of genes involved in beta cell function and communication. (A) Static GSIS test using primary mouse islets from WT and CX3CR1 KO mice fed NCD. Mean+/-SEM, n=6 per group. *, P<0.05. (B) Static GSIS test using primary mouse islets from WT and CX3CR1 KO mice fed HFD for 10 weeks. Mean+/-SEM, n=6 per group. (C) Static GSIS in the presence or absence of arginine (Arg; 10mM) using primary mouse islets. (D) Knockdown of CX3CR1 decreases GSIS in Min6 cells. Min6 cells were transfected with scrambled siRNA (Scrb) or 2 different CX3CR1 siRNAs (CX-1 and CX-2). 48h after transfection, GSIS was measured in the presence or absence of 100ng/ml FKN (left panel), or quantitative realtime RT-PCR was performed for CX3CR1 expression (right panel). **, P<0.01; ***, P<0.001. (E) Perifusion experiment using islets from WT and CX3CR1 KO mice on NCD. Mean+/-SEM, n=5 per group. *, P<0.05; **, P<0.01. (F-G) STZ-treated mice transplanted with CX3CR1 KO islets are more glucose intolerant than mice transplanted with WT islets. Plasma glucose (F) and insulin (G) levels during GTTs. Mean+/-SEM, n=6 per group. n=5 per group. (H) mRNA level of genes involved in beta cell function and communication in islets from WT or CX3CR1 KO mice either on chow and HFD. mRNA level of each gene was normalized to 18S rRNA level in the same sample. Mean+/-SEM, n=6 per group. *, P<0.05 WT-NCD vs KO-NCD or WT-NCT vs WT-HFD; **, P<0.01 WT-NCD vs KO-NCD or WT-NCT vs WT-HFD; #, P<0.05 WT-HFD vs KO-HFD. See also Figure S2.
To test whether the insulin secretory defect in CX3CR1 KO islets is associated with altered gene expression, we performed quantitative RT-PCR analyses. As shown in Figure 3H, the KO islets expressed lower levels of genes involved in normal beta cell secretory function such as PDX-1, NeuroD, insulin, Glut2, and urocortin3. Moreover, CX3CR1 KO islets exhibited reduced expression of Connexin 36 (Figure 3H), a gap junction component involved in beta cell communication allowing the synchronization of islet responses to metabolic signals (Calabrese et al., 2003; Carvalho et al., 2010; Speier et al., 2007). Furthermore, expression of some components of ATP-dependent potassium channels (Kir6.2) or L-type voltage-gated calcium channels (Cav1.2 and Cav1.3) was reduced in CX3CR1 KO islets although this did not reach statistical significance (Figure S2). Thus, the insulin secretory defect in CX3CR1 KO islets is associated with reduced expression of genes involved in beta cell function and communication.
Histologic Studies of WT and CX3CR1 KO Islets
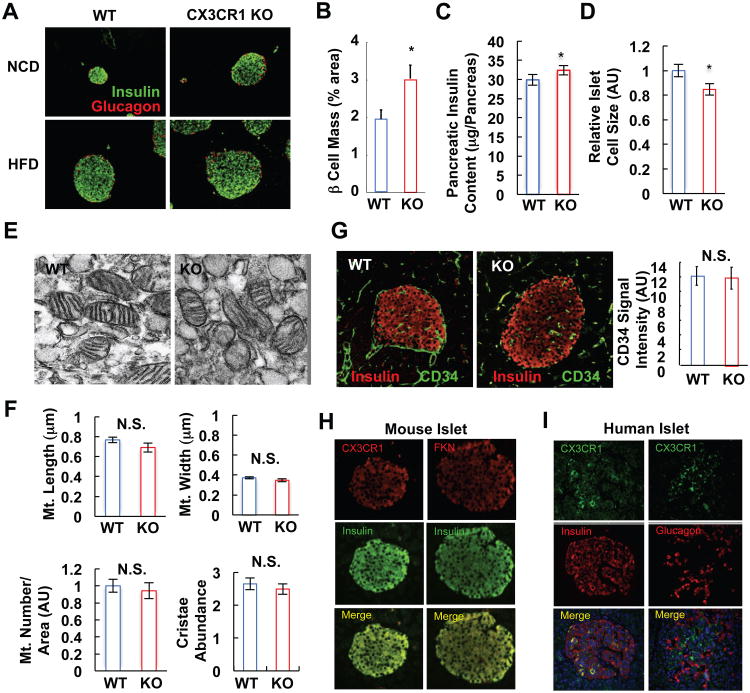
To determine whether CX3CR1 deletion affects islet development, we conducted immunohistologic (IHC) studies and found the expected effect of HFD to increase islet size (Maclean and Ogilvie, 1955; Pick et al., 1998). However, under both chow and HFD conditions, the islets were larger in the KO mice (Figure 4A). This was accompanied by a significant (P<0.05) increase in beta cell mass, as measured by morphometric analyses of insulin positive islet cells (Figure 4B), and a corresponding increase in total pancreatic insulin content (Figure 4C)(Figures S3A and S3B). These results show that CX3CR1 KO mice have sufficient beta cells and insulin, indicating that the in vivo phenotype is due to a defect in coupling extracellular signals to the insulin secretory machinery. Interestingly, the average beta cell size was decreased in the KO islets (Figure 4D), showing that the enhanced islet mass was due to an increased number of smaller beta cells. It has been reported that smaller beta cells display decreased GSIS activity (Giordano et al., 1993; Pende et al., 2000). Consistent with this, when we selected similar sized WT and CX3CR1 KO islets for comparison, a small but significant decrease in insulin content per islet was seen in the CX3CR1 KO group (Figure S3C).
Figure 4.
CX3CR1 KO mice exhibit increased beta cell mass and insulin content. (A) Immunohistochemistry analysis of WT and CX3CR1 KO islets using anti-insulin (green) and anti-glucagon (red) antibodies. (B) Beta cell mass of WT and CX3CR1 KO mice on NCD. Mean+/-SEM, n=8 per group. *, P<0.05. (C) Pancreatic insulin content. Mean+/-SEM, n=10 per group. (D) Relative islet cell size of WT and CX3CR1 KO mice. Relative islet cell size was calculated by dividing beta cell area by nuclei number. AU, arbitrary unit. Mean+/-SEM, n=8 per group. (E-F) Ultramicroscopic analysis of WT and CX3CR1 KO beta cells. (E) Ultramicroscopic pictures of WT and CX3CR1 KO mouse islets on NCD. (F) Mitochondrial length (upper left) and width (upper right), mitochondrial number per given area (lower left), and cristae abundance (lower right) was calculated using ImageJ software. Mean+/-SEM. 10 different EM pictures of WT and KO islets, and at least 2 mitochondria located nearest to the center of each EM picture were analyzed for the morphometry. AU, arbitrary unit; N.S., not significant. (G) Vascular density in the islets of WT and CX3CR1 KO mice on NCD was analyzed by immunohistochemistry. Pancreatic sections were co-stained with anti-CD34 (endothelial cell; green) and anti-insulin (beta cell; red) antibodies, and the intensity of CD34-positive signals in the insulin-positive area was measured and graphed in the right panel. Mean+/-SEM, n=6 (WT) and 8 (KO). (H) Immunohistochemistry analysis of mouse islets using anti-CX3CR1 (red, on the left), anti-FKN (red, on the right), or anti-insulin (green) antibodies. (I) Immunohistochemistry of human islet using anti-CX3CR1 (green) and anti-insulin (red), or anti-glucagon (red) antibodies. See also Figure S3.
The histologic analysis also showed that the morphologic features of the WT and KO islets, such as predominance of insulin positive beta cells in the core mantled by glucagon positive alpha cells, was unaffected by the KO (Figure 4A). To assess mitochondria in CX3CR1 KO islets, we performed ultramicroscopic analysis using electron microscopy. As seen in Figures 4E and 4F, morphology, size, number, and cristae abundance of mitochondria were comparable in WT and CX3CR1 KO beta cells, consistent with the normal oxygen consumption rate in the CX3CR1 KO islets (Figure S2). Formation of a proper microvascular network within islets is essential for adequate insulin secretion (Eberhard et al., 2010; Lammert et al., 2003), and since FKN/CX3CR1 can modulate angiogenic pathways, we assessed vascular density within the islets, by staining for the endothelial marker CD34. As seen in Figure 4G, CD34 staining was the same in CX3CR1 KO and WT islets. Finally, total pancreas mass in chow-fed or HFD CX3CR1 KO mice was comparable to WT (Figure S3). Together, the results suggest that the defective insulin secretion in CX3CR1 KO mice was not due to gross developmental defects, mitochondrial dysfunction, or defective intra-islet vascularization.
As shown in Figure 4H, IHC revealed that FKN and CX3CR1 are highly expressed in insulin-positive beta cells in mouse islets. Similarly, in human islets, CX3CR1 was expressed in beta cells, but not in glucagon-positive alpha cells (Figure 4I). RT-PCR analyses revealed that both the receptor and FKN are expressed in isolated human and murine islets, as well as in the Min6 (mouse) and INS-1 (rat) beta cell lines (Figure S3D).
In Vitro and In Vivo FKN Treatment
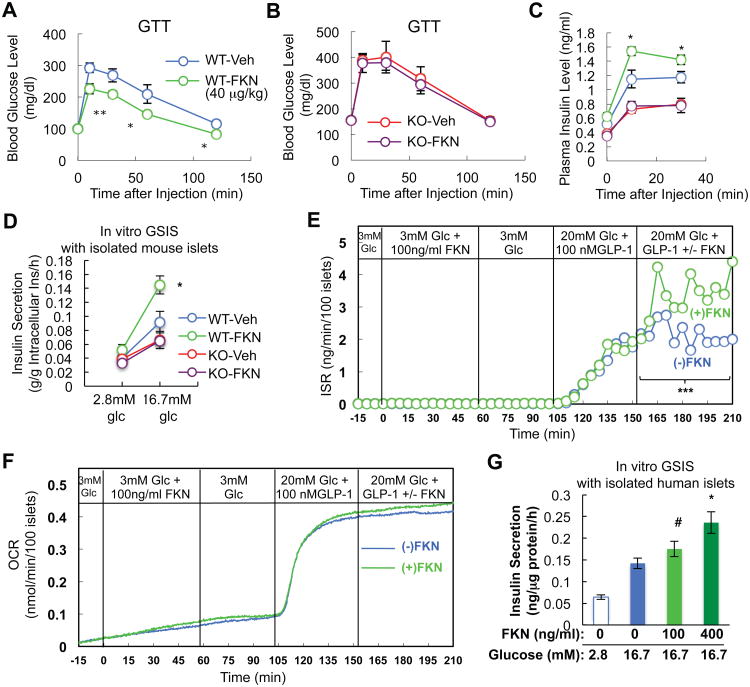
To provide a further test of our overall concept, we treated WT and CX3CR1 KO mice with acute administration of the 84 amino acid soluble chemokine portion of circulating mouse FKN. This led to improved glucose tolerance in the WT mice (Figure 5A), but was without effect on glucose levels in the KO animals (Figure 5B). Importantly, in vivo FKN administration caused an increase in insulin secretion in the WT mice, but not in the KOs (Figure 5C)(Figure S4). We also treated isolated WT and CX3CR1 KO islets with soluble FKN and found a marked 58% increase in GSIS in WT islets with no effect in the KO islets (Figure 5D). FKN also significantly potentiated glucose plus GLP1-induced insulin secretion in perifused primary mouse islets (Figure 5E), but did not effect oxygen consumption (Figure 5F). These in vivo and in vitro results demonstrate that FKN effects are direct and are CX3CR1-dependent. To demonstrate the translatability of these findings to human beta cells, we conducted studies in isolated human islets. As seen in Figure 5G, treatment of human islets with human FKN led to a dose responsive increase in GSIS with a 65% increase at the maximal concentration. Thus, the potentiating effects of FKN are quantitatively similar in mouse and human islets.
Figure 5.
FKN enhances insulin secretion and improves glucose tolerance in mice in a CX3CR1-dependnet manner. (A-C) WT and CX3CR1 KO mice (on NCD) were injected IP with glucose (2 g/kg) + FKN (40 μg/kg) or glucose + vehicle solution, and blood glucose and insulin levels were measured at the indicated time points. Mean+/-SEM, n=6 (vehicle) or 7 (FKN). (A) Glucose tolerance test in WT mice. *, P<0.05; **, P<0.01. (B) Glucose tolerance test in CX3CR1 KO mice. (C) Plasma insulin level during the GTTs. (D) In vitro static GSIS studies using WT and KO islets in the presence or absence of mouse FKN (100 ng/ml). n=6 per group. (E) Perifusion experiment using primary mouse islets in response to the indicated levels of glucose, GLP1 (100 nM) and FKN (100 ng/ml). ***, P<0.001. (F) Oxygen consumption rate in islets during the perifusion experiment in panel E. (G) In vitro static GSIS studies using human islets in the presence or absence of human FKN (100 or 400 ng/ml). n=4. #, P=0.076; *, P<0.05 compared with lane 2. See also Figure S4.
Effects of FKN Treatment on Beta Cell Signaling
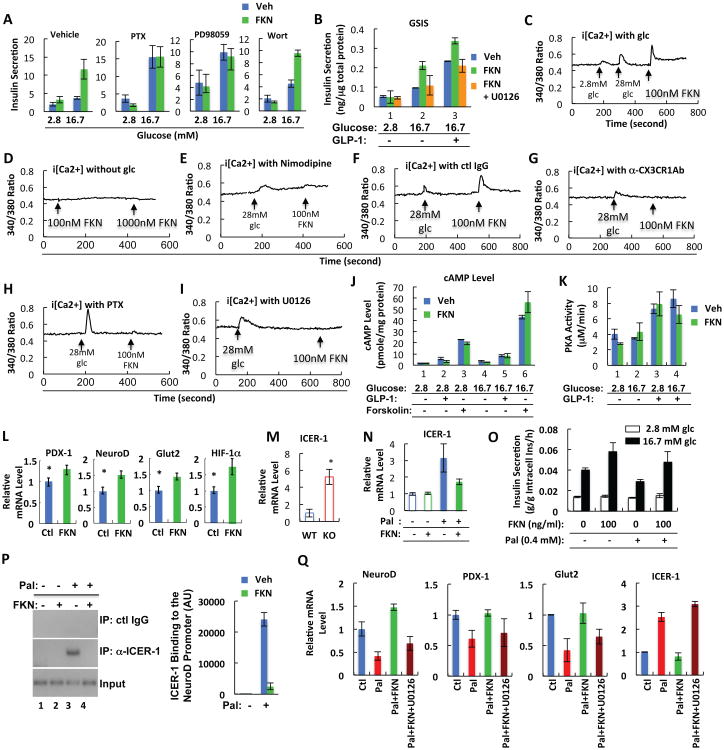
To determine the pathways by which FKN/CX3CR1 regulates insulin secretion, we measured GSIS in isolated islets treated with inhibitors of Gαi (pertussis toxin), PI3K (Wortmannin), and MEK (PD98059) at low and high glucose levels. FKN-stimulated insulin secretion was inhibited by pertussis toxin and the MEK inhibitor, but not by Wortmannin (Figure 6A). Consistent with these results, FKN stimulated ERK phosphorylation in a Gαi and MEK dependent fashion (Figure S5), and inhibition of MEK suppressed FKN potentiation of glucose and GLP1-induced insulin secretion in Min6 cells (Figure 6B). As seen in Figures 6A and 6B, FKN-stimulated insulin secretion was not evident at 2.8mM glucose and only occurred when sufficient glucose was provided (16.7mM), consistent with Figure 5, which shows that FKN is not a direct insulin secretagogue, but rather, potentiates the effects of other insulin secretory signals such as glucose and GLP1.
Figure 6.
FKN stimulates insulin secretion by increasing intracellular calcium levels in a CX3CR1- and MEK-dependent manner. (A) GSIS test using primary mouse islets with or without pertussis toxin (PTX; 250 ng/ml), Wortmannin (Wort; 10 μM), or PD98059 (50 μM), in the presence or absence of FKN (100 ng/ml). Mean+/-SEM, n=6 per group. (B) GSIS test using Min6 cells with or without FKN (100 ng/ml) or U0126 (10 μM). Mean+/-SEM. (C-I) Intracellular calcium level in Min6 mouse beta cells in the presence or absence of glucose (C, with glucose; D, without glucose), nimodipine (10 μM) (E), control antibody (F), anti-CX3CR1 neutralizing antibody (G), PTX (250 ng/ml) (H), or U0126 (10 μM) (I). Mean+/-SEM. (J) Intracellular cAMP level. Min6 cells were pre-incubated with isobutylmethylxanthine for 30 min, and then treated with GLP1 (100 nM), forskolin (100 μM), or FKN (100 ng/ml) at low (2.8 mM) or high (16.7 mM) glucose conditions for 30 min. Mean+/-SEM. (K) PKA enzymatic activity was measured in Min6 cells incubated in a low (2.8 mM) or high (16.7 mM) glucose condition for 15 min in the presence or absence of GLP1 (100 nM) or FKN (100 ng/ml). Mean+/-SEM. (L) FKN stimulates expression of genes involved in beta cell function. Primary mouse islets were incubated for 7 days with or without FKN (100 ng/ml), and mRNA expression of PDX-1, NeuroD, Glut2, and HIF-1α was measured by quantitative realtime RT-PCR. Mean+/-SEM. *, P<0.05. (M) ICER-1 mRNA expression in WT and CX3CR1 KO islets. Mean+/-SEM. (N) Palmitate-induced ICER-1 expression is suppressed by FKN in Min6 cells. Mean+/-SEM. (O) GSIS by Min6 cells treated with palmitate (Pal; 0.4 mM) for 48 h in the presence or absence of FKN (100 ng/ml). (P) FKN represses binding of ICER-1 to NeuroD promoter. Min6 cells were incubated in serum free media in the presence or absence of palmitate (100 μM) and/or FKN (100ng/ml). After 48h, the cells were fixed and subjected to chromatin immunoprecipitation with anti-ICER-1 antibody. (Q) Suppressive effect of FKN on ICER-1 expression is abolished by MEK inhibitor (U0126). Min6 cells were incubated with palmitate (Pal; 0.4 mM) in the presence or absence of FKN (100 ng/ml) or U0126 (10 μM). 48 h later, cells were harvested and subjected to quantitative realtime RT-PCR. See also Figures S5, S6 and S7.
In beta cells, calcium signaling is a critical component of the insulin secretory process (Seino et al., 2011). Since FKN has been shown to increase cytoplasmic calcium levels in macrophages and fibroblasts (Fong et al., 2002; Imai et al., 1997), we measured the FKN effect on intracellular calcium at low or high glucose conditions in Min6 cells. As shown in Figures 6C and 6D, FKN increased intracellular calcium levels with high glucose (6C), but was without effect in the absence of glucose (6D). Interestingly, inhibition of calcium influx by the L type calcium channel inhibitor, nimodipine, blocked both glucose- and FKN-induced intracellular calcium increase, implying that calcium influx is necessary for the FKN effect (Figure 6E). CX3CR1 neutralization with a specific antibody blocked the FKN-induced intracellular calcium increase, showing that this effect of FKN is mediated by CX3CR1 activation (Figures 6F and 6G). Moreover, the effect of FKN on intracellular calcium was blocked by treatment with the pertussis toxin or the MEK inhibitor (Figures 6H and 6I), suggesting that a Gai and MEK-mediated effect plays a mechanistic role in FKN-stimulated insulin secretion. Interestingly, the MEK inhibitor attenuated arginine-induced intracellular Ca2+ increase and insulin secretion in Min6 cells, but only in the presence of glucose (Figure S6).
Part of the effect of glucose and GLP1 to augment insulin secretion involves increases in cyclic AMP levels. Consequently, we measured glucose, GLP1, and forskolin stimulated cyclic AMP levels with and without FKN in Min6 cells (Figure 6J). Interestingly, FKN was without effect on cyclic AMP concentrations, and consistent with this, PKA activity was also unaffected by FKN treatment (Figure 6K).
We also assessed longer term effects of FKN on islet gene expression. 7 days of chronic in vitro treatment of islets with FKN led to increased expression of PDX1, NeuroD, HIF-1α and insulin (Figure 6L), and these genes were all down regulated in the CX3CR1 KO islets (Figure 3H). Recently, it has been shown that beta cell dysfunction induced by HFD or free fatty acid treatment, is, at least partially, mediated by the induction of inducible cyclic AMP early repressor (ICER-1) (Cho et al., 2012; Favre et al., 2011; Hussain et al., 2000; Zhou et al., 2003). Interestingly, ICER-1 expression was highly induced in the CX3CR1 KO islets (Figure 6M) and FKN treatment abolished palmitate-induced induction of ICER-1 mRNA in Min6 cells (Figure 6N). Concomitantly, FKN treatment prevented chronic palmitate-mediated inhibition of GSIS (Figure 6O). Moreover, using chromatin immunoprecipitation experiments, we found that FKN treatment blocked the effect of palmitate to induce binding of ICER-1 to the NeuroD promoter in Min6 cells (Figure 6P). These results suggest that one aspect of the FKN effect on beta cells might involve regulation of genes necessary for the insulin secretory machinery and this could be partially mediated by ICER-1 suppression. To test whether FKN regulation of ICER-1 is associated with MEK signaling pathways, we incubated Min6 cells with palmitate in the presence or absence of FKN and the MEK inhibitor. As shown in Figure 6Q, FKN treatment suppressed palmitate-induced ICER-1 expression while it increased expression of NeuroD, PDX-1 and Glut2; all of the FKN effects were abolished by the MEK inhibitor. These results suggest that FKN suppresses ICER-1 expression through a MEK-dependent pathway.
Beta cell failure in type 2 diabetes is associated with loss of beta cell volume as well as loss of GSIS (Prentki and Nolan, 2006). FKN has been shown to increase survival of microglial cells and vascular smooth muscle cells through a CX3CR1-PI3K-Akt-dependent pathway (Boehme et al., 2000; Chandrasekar et al., 2003). Therefore, we assessed whether FKN has in vitro effects on beta cell growth and survival. As shown in Figure S7A, FKN prevented beta cell apoptosis induced by chronic palmitate treatment, and this effect was comparable to that observed with GLP1 treatment. Moreover, FKN increased the number of viable cells, which was reduced by chronic palmitate treatment (Figures S7B and S7C). Interestingly, the increase in viable cell number by FKN was comparable to the degree of inhibition of beta cell apoptosis, suggesting that the increase of beta cell number was due to reduced apoptosis, rather than increased proliferation. Consistent with this, CX3CR1 KO did not change the number of Ki67 positive proliferating cells on both chow and HFD (Figure S7D). FKN stimulated Akt phosphorylation, which was inhibited by Wortmannin (Figure S7E). Moreover, the preventative effect of FKN against palmitate-induced apoptosis was inhibited by Wortmannin (Figure S7F), suggesting that the anti-apoptotic effect of FKN operates through a PI3K-Akt-dependent mechanism.
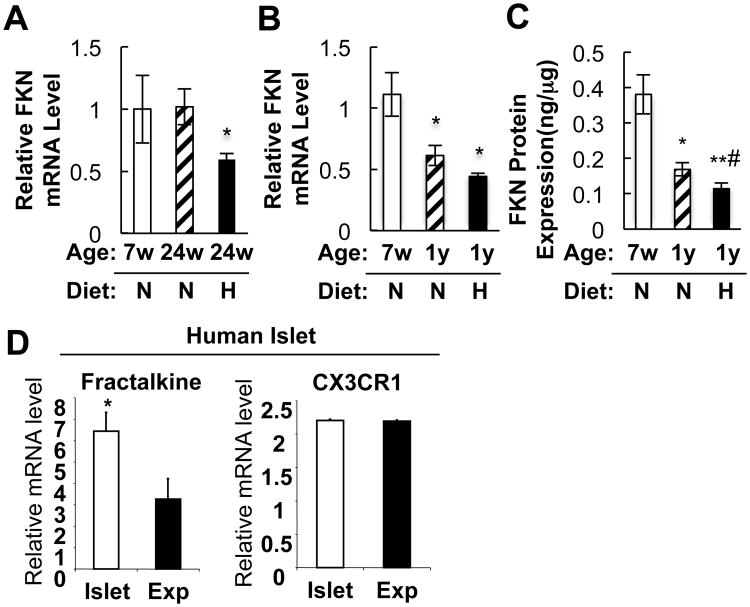
Islet FKN expression is decreased by aging and HFD
Aging and obesity are major risk factors for beta cell dysfunction. We found that mRNA and protein levels of FKN were decreased in islets from old or HFD/obese/diabetic mice (Figures 7A to 7C). On the other hand, CX3CR1 expression was not affected by aging or HFD (data not shown). Interestingly, FKN expression was not decreased after 24 weeks of age on normal chow, but was significantly decreased in the same age mice by 16 weeks of HFD, suggesting that HFD accelerates the decreased FKN expression in aging. Ex vivo expansion of beta cells causes dedifferentiation and loss of beta cell function. To evaluate whether reduced FKN/CX3CR1 signaling correlates with beta cell differentiation status, we compared FKN and CX3CR1 mRNA levels in human islets and ex vivo expanded human beta cells. As seen in Figure 7D, FKN, but not CX3CR1, expression was significantly decreased in expanded human islet-derived cells. We previously demonstrated that expanded human islet cells dedifferentiate and go through an epithelial to mesenchymal transition in vitro (Kayali et al., 2007; Kutlu et al., 2009), such that the resulting expanded cells are closer in phenotype to mesenchymal stem cells than to the original endocrine cells.
Figure 7.
FKN expression is decreased by aging and HFD in islets. (A) FKN mRNA expression in 7 week old (7w)-NCD (N), 24 week old (24w)-NCD, or 24 week old-HFD mice. *, P<0.05 24w-NCD vs 24w-HFD. FKN mRNA level was normalized to 18S rRNA level in each sample. Mean+/-SEM, n=6 per group. (B) FKN mRNA expression in 7 week old (7w)-NCD (N; n=5), 1 year old (1y)-NCD (n=8), or 1 year old-HFD (H; n=6) mice. Mean+/-SEM. *, P<0.05 7w-NCD vs 1y-NCD or 7w-NCD vs 1y-HFD. (C) FKN protein expression levels were measured in aliquots of the samples used in panel B. FKN protein levels were normalized by total protein concentration. Mean+/-SEM. *, P<0.05 7w-NCD vs 1y-NCD; **, P<0.01 7w-NCD vs 1y-HFD; #, P<0.05 1y-NCD vs 1y-HFD. (D) FKN mRNA expression is decreased in in vitro expanded human beta cells. mRNA levels of FKN and CX3CR1 were analyzed by Affymetrix GeneChip microarrays (Kayali et al., 2007; Kutlu et al., 2009) in freshly isolated human islets (Islet) or in vitro expanded islets (Exp) as described in experimental procedures. Mean+/-SEM.
Discussion
Here we describe a mechanism for regulating beta cell function through the FKN/CX3CR1 system. CX3CR1 KO mice develop glucose intolerance on both chow and HFD due to decreased insulin secretion. Neutralization of circulating FKN by in vivo administration of anti-FKN antibodies recapitulated this effect, causing glucose intolerance with diminished insulin secretion, demonstrating that FKN is necessary for ongoing maintenance of circulating insulin levels. The impaired insulin secretion represents a primary beta cell defect, since isolated islets from CX3CR1 KO mice exhibited impaired GSIS compared to WT islets and CX3CR1 KO islets transplanted into STZ-induced diabetic mice had an attenuated ability to correct the diabetic state compared to transplantation of WT islets. Finally, in vivo administration of FKN treatment led to improved glucose tolerance with increased insulin secretion and in vitro treatment directly caused increased GSIS in isolated mouse and human islets. In contrast, FKN was without effect on insulin secretion in CX3CR1 KO mice or in islets from KO animals. Taken together, these studies reveal a regulatory system for beta cell insulin secretion, suggesting that a FKN-based biotherapeutic, or a small molecule CX3CR1 agonist, could be a useful therapeutic tool in the treatment of type 2 diabetes.
CX3CR1 is the unique receptor for FKN and FKN is the only known ligand for this G protein-coupled receptor (Imai et al., 1997; Zernecke et al., 2008). FKN is expressed as a membrane-bound protein, which can interact with CX3CR1 on adjacent cells to facilitate cell:cell adhesion and communication (Haskell et al., 1999; Zernecke et al., 2008). The extracellular domain of FKN can be cleaved through the action of the extracellular proteases Adam 10 and 17 to produce a soluble form of FKN (Garton et al., 2001; Hundhausen et al., 2003). Soluble FKN can exert paracrine effects in the extracellular space and can also enter the circulation to potentially cause endocrine effects on distant tissues (Shah et al., 2011).
It has recently been reported that two single nucleotide polymorphisms (T280M and V249I), located in the coding sequence of human CX3CR1, are associated with an increased incidence of Type 2 Diabetes and metabolic syndrome (Shah et al., 2011; Sirois-Gagnon et al., 2011). These CX3CR1 gene variants result in lower FKN binding affinity, consistent with the view that the FKN/CX3CR1 system plays a beneficial role in the maintenance of proper insulin secretion and glycemic control. On the other hand, circulating levels of soluble FKN are not decreased in Type 2 diabetic patients and, in fact, are slightly higher than controls (Shah et al., 2011). This raises the question as to why beta cell function is defective in the face of increased circulating FKN levels in Type 2 diabetes. Certainly, beta cell dysfunction in diabetes is a complicated, multi-factorial process, involving factors in addition to FKN/CX3CR1. However, several other possibilities also come to mind. First, it is possible that the local expression of soluble FKN is dominant over the circulating levels and that intra-islet FKN levels are low in Type 2 diabetes, similar to what we have observed in aging and HFD/obese islets in mice. Secondly, CX3CR1 signaling could be impaired in diabetic beta cells leading to FKN resistance. Finally, while FKN levels are not decreased, it is possible that CX3CR1 expression is decreased in beta cells from Type 2 diabetes patients, although we did not observe this in islets from aging or HFD/obese mice. Clearly, future studies will be necessary to explore these issues.
Our in vitro studies demonstrate that FKN is not a direct insulin secretagogue, since it does not enhance insulin secretion in the absence of glucose or at low glucose concentrations. Instead, FKN only exerts its effects by potentiating GSIS, arginine-, or GLP1-mediated insulin secretion. Our in vitro studies show that the acute effects of FKN on insulin secretion are not due to changes in mitochondrial function, cyclic AMP levels, or increased PKA activity. In the presence of glucose, FKN causes an increase in intracellular calcium levels through a MEK-dependent mechanism. Combined with the fact that CX3CR1 deletion impairs the insulin secretory response to arginine, this suggests that the effects of FKN on insulin secretion are exerted at a downstream step common to several stimulatory inputs, most likely involving MEK-dependent calcium mobilization events.
Our data demonstrate that FKN has acute effects to potentiate GSIS and GLP1-stimulated insulin secretion, but it is also possible that FKN has chronic effects on beta cell function as well. Thus, CX3CR1 KO islets display decreased expression of a set of genes characteristic of normal, fully functioning beta cells, including PDX1, NeuroD, GLUT2, urocortin3, and CX36. Furthermore, chronic FKN treatment of WT islets leads to increased expression of these genes. Thus, one could take the view that CX3CR1 deletion, with ablation of FKN signaling, produces beta cells that are partially dedifferentiated. Indeed, the KO islets are characterized by increased total cell mass due to increased numbers of smaller beta cells, and it has been reported that reduced beta cell size is associated with impaired insulin secretory function (Giordano et al., 1993; Pende et al., 2000). Along these lines, we found that ICER-1 is induced in CX3CR1 KO islets while FKN treatment of Min6 cells suppresses palmitate-induced ICER-1 expression. ICER-1 is a transcriptional repressor which can inhibit genes associated with the normal differentiated beta cell functional state. It has been shown that ICER-1 is induced by saturated fatty acids, oxidized LDL, hyperglycemia, and high fat diet and ICER-1 induction can cause beta cell dysfunction by inhibiting expression of CX36, as well as components of the insulin secretory machinery as exemplified in Figure 3 (Favre et al., 2011; Hussain et al., 2000; Zhou et al., 2003). This suggests that increased ICER-1 expression in CX3CR1 KO beta cells is mechanistically linked to a more chronic state of beta cell dysfunction. In this context, it is important to note that in vitro siRNA-mediated knockdown of CX3CR1 in Min6 cells caused decreased GSIS, suggesting that the in vivo decrease in insulin secretion in the KO mice was not due to an in vivo beta cell developmental defect.
Together, these studies describe a novel pathway regulating beta cell secretory function in which FKN stimulates CX3CR1 to promote increased insulin secretory responses. CX3CR1 deficiency mimics some of the beta cell abnormalities observed in diabetic islets and FKN treatment restores these defects towards normal. In mice, aging and the HFD/obese hyperglycemic state are associated with decreased islet FKN expression. Thus, attenuation of FKN/CX3CR1 system could potentially underlie some of the defects in diabetic islets. Furthermore, a FKN-based biotherapeutic, or a small molecule CX3CR1 agonist, could have potential utility in the treatment of type 2 diabetes.
Experimental Procedures
Animals and treatments
7 week old male C57BL/6N and CX3CR1 knockout mice were purchased from Taconic (USA). GTT and ITT results from the CX3CR1 KO mice were confirmed in CX3CR1gfp/gfp knockin mice obtained from Jackson Laboratory (USA). Oral glucose tolerance test and ip insulin tolerance test were performed as described previously (Lee et al., 2010; Lee et al., 2011). For arginine tolerance test, the mice were fasted for 6 h and basal blood samples were taken, followed by IP injection of 1g/kg arginine. All animal procedures were in accordance with the research guidelines for the use of laboratory animals of University of California, San Diego. Detailed protocols are described in Extended Experimental Procedures.
Plasma protein measurements
Plasma insulin (ALPCO, USA), C-peptide (ALPCO, USA), GLP1 (active 7-36 GLP1; Millipore, USA), and fractalkine (R&D systems, USA) levels were measured by ELISA.
Islet transplantation
A single aliquot of 200 freshly isolated islet equivalents was transplanted into the left kidney capsule of the recipient mice. For details, please see Extended Experimental Procedures.
Measurement of insulin secretory response from isolated islets
For dynamic resolution of insulin secretory response, islets were perifused at 37°C, 5% CO2 in a system comprised of a pump, gas equilibrating system and islet chamber, as described previously (Sweet et al., 2004). For static GSIS assays, approximately 20 mouse islets or 100 IEQ human islets were incubated for 2 hours in low glucose media at 37°C, 5%CO2, and then incubated for 60 min or 75min with 2.8mM or 16.7mM glucose in the same conditions. For details, please see Extended Experimental Procedures.
Intracellular calcium level measurement
The Cytoplasmic free calcium ion concentration in Min6 cells was measured by fura-2 fluorescence ratio digital imaging. For details, please see Extended Experimental Procedures.
cAMP level measurement
Intracellular cAMP level of Min6 cells was measured using Bridge-it cAMP assay kit (Mediomics, LLC, USA).
Electron microscopy
Electron microscopy. Scanning electron micrographs were taken using Phillips 12 XL30 ESEM in an environmental mode at Calit2-Nano3 facility. For details, please see Extended Experimental Procedures.
Human islet and in vitro beta cell expansion
The human islet and beta cell samples were prepared and analyzed as described previously (Kayali et al., 2007; Kutlu et al., 2009). Details are described in Extended Experimental Procedures.
Statistics
The results are shown as means ± SEM. All statistical analysis was performed by Student's t test or ANOVA in Excel (Microsoft); P < 0.05 was considered significant.
Supplementary Material
Highlights.
CX3CR1 KO mice exhibit impaired glucose tolerance due to reduced insulin secretion.
Isolated islets from CX3CR1 KO mice exhibit impaired insulin secretion.
Fractalkine treatment stimulates insulin secretion and improves glucose tolerance.
Fractalkine effect is mediated by increasing [Ca2+]i level and ICER-1 suppression.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants DK-033651, DK-074868, T32-DK-007494, and DK-063491; and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement of U54-HD-012303-25 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research. Y.S.L. was supported by a Mentor-Based Postdoctoral Fellowship Award from American Diabetes Association. Islet perifusions were carried out by the Diabetes and Endocrine Research Center Islet Core located at the University of Washington (P30 DK017047). We thank to Dr. Ulupi Jhala (University of California, San Diego) for providing Min6 cells with permission from Dr. Miyazaki Jun-Ichi (Osaka University). H.D. is co-founder of AddexBio. No potential conflicts of interest relevant to this article were reported.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52:1390–1400. doi: 10.1002/hep.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol. 2000;165:397–403. doi: 10.4049/jimmunol.165.1.397. [DOI] [PubMed] [Google Scholar]
- Calabrese A, Zhang M, Serre-Beinier V, Caton D, Mas C, Satin LS, Meda P. Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes. 2003;52:417–424. doi: 10.2337/diabetes.52.2.417. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carvalho CP, Barbosa HC, Britan A, Santos-Silva JC, Boschero AC, Meda P, Collares-Buzato CB. Beta cell coupling and connexin expression change during the functional maturation of rat pancreatic islets. Diabetologia. 2010;53:1428–1437. doi: 10.1007/s00125-010-1726-8. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Liu F, Melby PC. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. The Biochemical journal. 2003;373:547–558. doi: 10.1042/BJ20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IS, Jung M, Kwon KS, Moon E, Cho JH, Yoon KH, Kim JW, Lee YD, Kim SS, Suh-Kim H. Deregulation of CREB Signaling Pathway Induced by Chronic Hyperglycemia Downregulates NeuroD Transcription. PloS one. 2012;7:e34860. doi: 10.1371/journal.pone.0034860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D, Kragl M, Lammert E. ‘Giving and taking’: endothelial and beta-cells in the islets of Langerhans. Trends in endocrinology and metabolism: TEM. 2010;21:457–463. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Favre D, Niederhauser G, Fahmi D, Plaisance V, Brajkovic S, Beeler N, Allagnat F, Haefliger JA, Regazzi R, Waeber G, et al. Role for inducible cAMP early repressor in promoting pancreatic beta cell dysfunction evoked by oxidative stress in human and rat islets. Diabetologia. 2011;54:2337–2346. doi: 10.1007/s00125-011-2165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AM, Alam SM, Imai T, Haribabu B, Patel DD. CX3CR1 tyrosine sulfation enhances fractalkine-induced cell adhesion. The Journal of biological chemistry. 2002;277:19418–19423. doi: 10.1074/jbc.M201396200. [DOI] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) The Journal of biological chemistry. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Giordano E, Cirulli V, Bosco D, Rouiller D, Halban P, Meda P. B-cell size influences glucose-stimulated insulin secretion. The American journal of physiology. 1993;265:C358–364. doi: 10.1152/ajpcell.1993.265.2.C358. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. The Journal of biological chemistry. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Daniel PB, Habener JF. Glucagon stimulates expression of the inducible cAMP early repressor and suppresses insulin gene expression in pancreatic beta-cells. Diabetes. 2000;49:1681–1690. doi: 10.2337/diabetes.49.10.1681. [DOI] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. The Journal of biological chemistry. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Kayali AG, Flores LE, Lopez AD, Kutlu B, Baetge E, Kitamura R, Hao E, Beattie GM, Hayek A. Limited capacity of human adult islets expanded in vitro to redifferentiate into insulin-producing beta-cells. Diabetes. 2007;56:703–708. doi: 10.2337/db06-1545. [DOI] [PubMed] [Google Scholar]
- Kutlu B, Kayali AG, Jung S, Parnaud G, Baxter D, Glusman G, Goodman N, Behie LA, Hayek A, Hood L. Meta-analysis of gene expression in human pancreatic islets after in vitro expansion. Physiological genomics. 2009;39:72–81. doi: 10.1152/physiolgenomics.00063.2009. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Current biology: CB. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lee YS, Choi JW, Hwang I, Lee JW, Lee JH, Kim AY, Huh JY, Koh YJ, Koh GY, Son HJ, et al. Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. The Journal of biological chemistry. 2010;285:22174–22185. doi: 10.1074/jbc.M109.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. The Journal of clinical investigation. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. The American journal of pathology. 2001;158:855–866. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4:367–376. doi: 10.2337/diab.4.5.367. [DOI] [PubMed] [Google Scholar]
- Morris DL, Oatmen KE, Wang T, DelProposto JL, Lumeng CN. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring) 2012;20:1189–1199. doi: 10.1038/oby.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. The Journal of clinical investigation. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, obesity & metabolism. 2008;10(4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. The Journal of clinical investigation. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512–1518. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois-Gagnon D, Chamberland A, Perron S, Brisson D, Gaudet D, Laprise C. Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obesity (Silver Spring) 2011;19:222–227. doi: 10.1038/oby.2010.125. [DOI] [PubMed] [Google Scholar]
- Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes. 2007;56:1078–1086. doi: 10.2337/db06-0232. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis J, Reems J. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401–409. doi: 10.2337/diabetes.53.2.401. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. The Journal of clinical investigation. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;3(3):S16–21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- Zhou YP, Marlen K, Palma JF, Schweitzer A, Reilly L, Gregoire FM, Xu GG, Blume JE, Johnson JD. Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets. A common mechanism for glucose toxicity and lipotoxicity? The Journal of biological chemistry. 2003;278:51316–51323. doi: 10.1074/jbc.M307972200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.