Abstract
The yeast deletion library is a collection of over 5100 single gene deletions that has been widely used by the yeast community. The presence of a non-mendelian element, such as a prion, within this library could affect the outcome of many large-scale genomic studies. We previously showed that the deletion library parent strain contained the [PIN+] prion. [PIN+] is the misfolded infectious prion form of the Rnq1 protein that displays distinct fluorescent foci in the presence of RNQ1:GFP and exists in different physical conformations, called variants. Here, we show that over 97% of the library deletion strains are [PIN+]. Of the 141 remaining strains that have completely (58) or partially (83) lost [PIN+], 139 deletions were able to efficiently maintain three different [PIN+] variants despite extensive growth and storage at 4°C. One strain, cue2Δ, displayed an alteration in the RNQ1:GFP fluorescent shape, but the Rnq1p prion aggregate shows no biochemical differences from the wildtype. Only strains containing a deletion of either HSP104 or RNQ1 are unable to maintain [PIN+], indicating that 5153 non-essential genes are not required for [PIN+] propagation.
Introduction
The single-gene disruption collection (Winzeler et al., 1999) has provided the yeast community with a powerful tool to perform genomic studies. The library consists of over 5,100 strains, each carrying a non-essential gene replaced with a KanMX cassette and a unique 20 nucleotide identification sequence or “bar-code.” Over the last decade, this collection has been employed for many large scale studies, including: synthetic lethality (Tong et al., 2001), functional analysis (Breslow et al., 2008) and drug screens (Chang et al., 2002; Giaever et al., 2004; Hillenmeyer et al., 2008); as well as for asking specific questions such as identifying genes that affect aging (Powers et al., 2006) and prion toxicity (Tyedmers et al., 2008).
The unknown presence of an epigenetic or non-Mendelian element could impact the outcome of large-scale genetic screens. We had earlier reported that the parent strain of the deletion library, BY4741, contained a prion called [PIN+] (Derkatch et al., 2001), also referred to as [RNQ+] (Sondheimer and Lindquist, 2000), which is an aggregated, self-perpetuating form of the Rnq1 protein (Derkatch et al., 2001). The [PIN+] prion has been shown to enhance the appearance of the prion form of Sup35p, called [PSI+]. Both prions require the non-essential Hsp104p chaperone for propagation (Chernoff et al., 1995; Sondheimer and Lindquist, 2000).
The same prion proteins can exist in different conformations, which have been referred to as “strains” or variants (reviewed in Bruce, 2003; and Derkatch et al., 1996). Yeast prion variants not only display different conformational characteristics, but also different phenotypes and stabilities (Derkatch et al., 1996; Derkatch et al 1997; Schlumpberger et al., 2001; Bradley et al., 2002; Kryndushkin et al., 2003; Bagriantsev and Liebman, 2004; King and Diaz-Avalos, 2004; Tanaka et al., 2004; Diaz-Avalos et al., 2005; Krishnan and Lindquist, 2005; Toyama et al., 2007). Furthermore, prion variants can affect the stability of another prion. For example, certain [PSI+] variants become unstable in the presence of certain [PIN+] variants (Bradley and Liebman; 2004).
Since the parent strain of the MATa deletion library contained [PIN+], we set forth to identify all strains in the collection that retained the [PIN+] prion. Those strains that were [pin−] were further tested for their ability to propagate the prion by cytoducing them with [PIN+]. We found that over 97% of the library contained [PIN+], while 58 strains appeared to be [pin−] and 83 strains had partially lost [PIN+]. When the same [PIN+] or different variants of [PIN+] were introduced into these 141 strains, only rnq1Δ and hsp104Δ failed to propagate these prions. Thus, the other 5153 non-essential genes present in the library are not required for the propagation of the [PIN+] prion.
Materials and methods
Plasmids and strains
The yeast deletion library (parent strain BY4741: MATa his3Δ1 leu2Δ ura3Δ met15Δ; Open Biosystems, Huntsville, AL; Cat # YSC1053) was screened for the [PIN+] prion. To detect [PIN+], a plasmid containing a copper inducible RNQ1 gene fused to GFP (p1186; CEN LEU2 ori ARS AmpR pCup1-RNQ1:GFP, called pCUP1-RNQ1:GFP) was used (kind gift from S. Lindquist, Whitehead Institute; Sondheimer and Lindquist, 2000; Derkatch et al., 2001). The “tester strain” was made by curing L2174 (MATα leu2 ura2 his3 [pin−]) on GuHCl (see below) to eliminate all known prions, and transforming the cured strain with pCUP1-RNQ1:GFP. In the “kar1 plasmid donor” strain (J1362; MATα CEN1–16::pGal1-CEN1–16-URA3Kl kar1Δ15 lys2 rad5-535 leu2-3,112 can1-100 his3-11,15 trp1-1 cyhR; R. J. D. R. and R. R., unpublished), the centromere on every chromosome is tagged with the K. lactis URA3 gene and contains the GAL1 promoter that disrupts each centromere’s function when activated (Reid at al., 2008; Chlebowicz-Sledziewska and Sledziewski, 1985; Hill and Bloom, 1987).
Cultivation procedures
Saccharomyces cerevisiae strains were propagated using standard media and cultivation protocols (Sherman et al., 1986). Except in the case of the kar1 plasmid donor, which was grown at room temperature prior to mating with the yeast disruption library, all cells were grown at 30°C. Complex media contained 2% dextrose (YPD) or 2% glycerol (YPG). Synthetic complete media contained the required amino acids and 2% dextrose (SD) or 2% galactose (Gal). Strains transformed with pCUP1-RNQ1:GFP were maintained on synthetic complete media lacking leucine (−Leu). To select against cells expressing URA3, cells were grown on 5′ fluoroorotic acid (5′ FOA) media, made according to Rose et al. (1990).
Scoring for [PIN+] in the Yeast Disruption Library
Library deletion strains were pinned onto YPD containing a lawn of the tester strain (see above, “plasmids and strains”). The library and tester cells were then allowed to mate overnight. Diploids containing the plasmid were selected on −Leu-Ura media. The pCUP1-RNQ1:GFP plasmid was induced by transferring diploids into 400 μL of −Leu-Ura plus 50μM copper sulfate liquid media and incubating for 16 hours without shaking. Cells were then manually transferred to 15 well glass slides and observed using a Zeiss Axioskop2 deconvolution workstation equipped with either a X40 Plan-Neofluar or X100 Plan-Apochromat objective lens (Zeiss). Deletion strains were individually scored for the presence of either cytoplasmic diffuse fluorescence ([pin−]) or fluorescent aggregates ([PIN+]). Library strains that initially showed a population containing less than 100% of fluorescing cells with aggregates were reconfirmed by mating to two independently transformed pCUP1-RNQ1:GFP containing tester strains and re-checked for aggregation.
Curing of library strains
Strains were cured either by spotting or streaking onto YPD containing 5mM guanidine HCl (GuHCl) where they were grown for three days. GuHCl is a chaotropic agent that cures strains of known prions through the inactivation of HSP104 (Tuite et al., 1981; Jung and Masison, 2001). To increase the probability that all prions were cured, strains were subjected to three more passes on GuHCl media and were then transferred to YPD media.
Preparation of the kar1 plasmid donor strain
Cytoduction was used to transfer prion-containing cytoplasm from the kar1 plasmid donor to recipient library cells. Appropriate prions and plasmids were introduced into the kar1 plasmid donor strain using cytoduction as described below: a [rhoo] version of the kar1 plasmid donor was mated with [RHO+] strains containing medium [PIN+] (L2257), high [PIN+] (L2262) (Bradley et al., 2002; Derkatch et al., 1996) or the “library” [PIN+] (lys2Δ; YBR115C) from the BY4741 disruption library. Cytoductants were selected on YPG containing 10 μg/ml cycloheximide, which selects for the cyhR [RHO+] version of the kar1 plasmid donor. Cytoductants were confirmed by testing for auxotrophic markers and the presence of the prion was verified by checking for the formation of RNQ1:GFP aggregates as described above.
Introduction of prions into candidate strains using the kar1 plasmid donor
While cytoduction has been a routine means to transfer prions from one strain to another, cytoduction of prions into many strains has proven very labor intensive. To overcome this obstacle, a kar1 plasmid donor strain was used. This strain was developed to improve cytoduction-based plasmid transfer by adding a counter-selectable marker to every chromosome (Georgieva and Rothstein, 2002 and R. J. D. R. and R. R., unpublished). The kar1 plasmid donor strain contains a kar1Δ15 mutation that permits mating and mixture of the cytoplasm, but prevents fusion between the nuclei of donor and recipient strains (Conde and Fink, 1976). In addition, each chromosome of the kar1 plasmid donor strain contains a conditional centromere in which a wild type K. lactis URA3 gene and an adjacent GAL1 promoter has been cloned next to each centromere (Reid et al., 2008). To perform cytoduction, a lawn of the kar1 plasmid donor (containing a pCUP1-RNQ1:GFP LEU2 plasmid and appropriate prions) was grown at room temperature for 16 hours. Deletion library candidates were pinned onto these lawns and cells were allowed to mate for six to eight hours. Short mating times were essential to limit the propagation of diploids in subsequent steps. Mating mixtures replica plated onto Gal-Lys-Leu were allowed to grow for 5 to 7 days (Fig. 1). The −Leu selects for the plasmid, −Lys selects against the donor, and the galactose destabilizes the conditional chromosomes in the heterokaryon and diploid cells. Since one transfer to Gal-Lys-Leu still permitted the propagation of some kar1 plasmid donor and diploid cells, the cells were spotted onto Gal-Lys-Leu media again. The spotting procedure used involves transferring the cells with a 48 or 96 pronged spotter into 300 μL of sterile water, resuspending and then spotting the cells on the appropriate media. Occasionally, chromosomes from the donor are transmitted to recipient strains. To avoid this contamination, strains were spotted on Gal-Lys-Leu supplemented with 5′ fluoroorotic acid (5′ FOA) for 2 days. Since the Ura3 protein converts 5′ FOA to the toxic 5′ fluorouracil compound (Boeke et al., 1984), cytoductants possessing any URA3 donor chromosomes will die. To ensure that all kar1 donor strain chromosomes were eliminated, cells were spotted to SD-Lys-Leu plus 5′ FOA and grown for an additional two days, and then maintained on SD-Leu media. While the majority of cells are cytoductants, some cells could be derived from diploids in which the donor chromosomes were destabilized. All cytoductions were performed in duplicate with independently cytoduced kar1 donors.
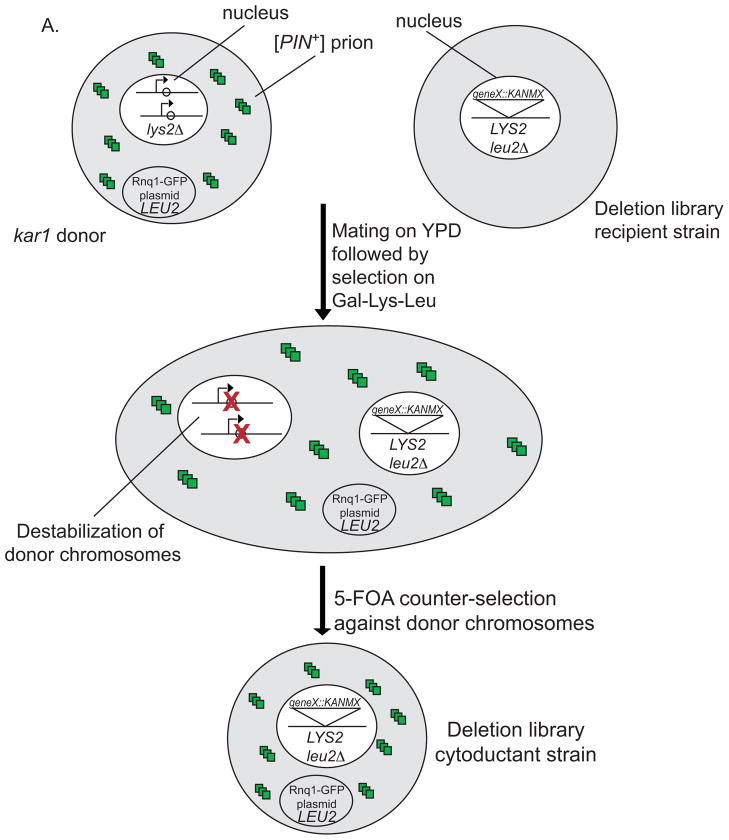
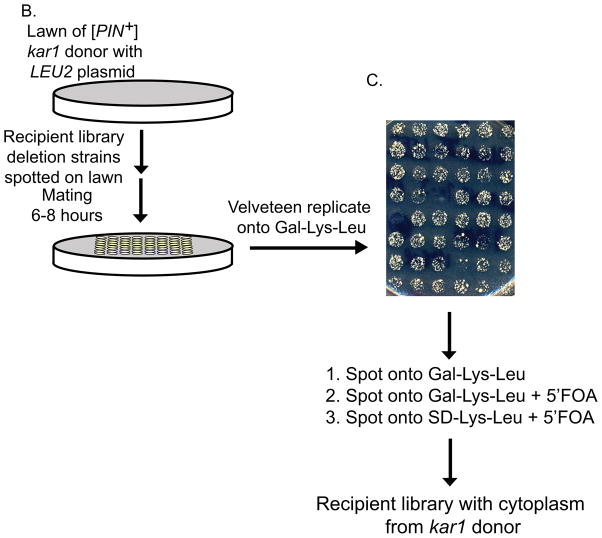
Figure 1. Cytoduction using the kar1 plasmid donor.
A. Each chromosome of the kar1 plasmid donor (top left) contains a wild type K. lactis URA3 gene and a neighboring GAL1 promoter that has been cloned adjacent to the centromere (arrow, Reid et al., 2008). The [PIN+] (small squares) lys2Δ kar1 plasmid donor that contains the LEU2 RNQ1:GFP plasmid forms heterokaryons (center) when mated to the LYS2 leu2Δ deletion library recipient strain (top right). While the cytoplasmic contents of donor and recipient cells mix, nuclear fusion is inhibited by the presence of the kar1Δ15 allele in the donor nucleus (see materials and methods). Upon plating on Gal-Lys-Leu, transcription from the GAL1 promoter through the centromeres destabilizes the chromosomes from the donor nucleus while −Lys selects against the donor cells and −Leu selects for the plasmid originally in the donor cell. Successive growth on media containing 5′ FOA (see part C) selects against any remaining chromosomes from the donor. The resulting cytoductants contain the Lys+ recipient nucleus with no donor cell chromosomes and the LEU2 RNQ1:GFP plasmid from the donor cell (bottom). B) A thin lawn of the kar1 plasmid donor was grown overnight, and the candidate strains were directly pinned onto the lawn and mated for six to eight hours. C). Mated cells were velveteen replicated onto Gal-Leu-Lys media and allowed to grow for five to seven days. While pilot studies have shown that the majority of cells within the spot are cytoductants, small amounts of donors and diploids were detected (data not shown). To eliminate remaining donors and diploids, cells were spotted again on Gal-Leu-Lys, and then spotted onto Gal-Leu-Lys+FOA followed by SD-Leu-Lys+FOA to remove any cytoductants carrying donor chromosomes from the population.
Determining the presence of [PIN+] in strains that are unable to mate
A handful of strains were unable to mate with either the tester strain or the kar1 plasmid donor (Table 1), many of which have been reported to be sterile or are MATα instead of being MATa. Therefore, these strains were directly transformed with the pCUP1-RNQ1:GFP plasmid, induced in 400 μL of −Leu supplemented with 50μM copper sulfate and manually observed for aggregates in order to detect the presence of preexisting [PIN+].
Table 1.
Library deletion strains that were directly transformed and tested for [PIN+].
| YBL033C (RIB1) | YDR461W (MFA1)** | YHL004W (MRP4) | YJR114W |
| YBR020W (GAL1) | YEL004W (YEA2)* | YHL030W (ECM29)* | YKL135C (APL2)* |
| YBR112C (CYC8) | YEL029C (BUD16)* | YHR175W-A | YLR362W (STE11)** |
| YDL041W** | YEL072W (RMD)* | YHR177W* | YML007C-A |
| YDL042C (SIR2)** | YER115C | YIL009W (FAA3) | YOL086C (ADH1)* |
| YDL160C-A | YFL025C (BST1)* | YIL069C (RPS24b)* | YOR212W (STE4)** |
| YDR079C-A (TBF5) | YGL007C-A | YJL096W (MRPL49) | YPL006W (NCR1)* |
| YDR103W (STE5)** | YGR037C (ACB1) | YJR005C-A | |
| YDR227W (SIR4)** | YHL003C (LAG1)* | YJR086W (STE18)** |
deletion strain was MATα
sterile deletion
Results
Screen of deletion library strains for the [PIN+] prion
The [psi−][pin−] pCUP1-RNQ1:GFP tester strain was mated to each of the 5155 library deletion strains. Expression of the RNQ1:GFP fusion was induced in the resulting diploids, which were examined microscopically for fluorescent aggregates, indicative of the [PIN+] prion. Strains that were unable to mate (Table 1) were transformed with the pCUP1-RNQ1:GFP plasmid and assayed for [PIN+]. The majority of strains appeared to contain essentially all [PIN+] cells. However, 58 strains displayed completely diffuse cytoplasmic fluorescence, consistent with being [pin−] (Table 2). This class included two genes that are known to be required for [PIN+] maintenance, RNQ1 and HSP104. In addition, 83 strains had a mixed population of cells having both diffuse and punctate fluorescence (Table 3).
Table 2.
Library deletion strains that do not contain [PIN+]
| YAL031C (GIP4) | YDL117W (CYK3) | YGL218W | YLR046C |
| YAL046C (AIM1) | YDL200C (MGT1) | YGL251C (HFM1) | YLR047C (FRE8) |
| YAL048C (GEM1) | YDR290W | YGR011W | YLR278C |
| YAL056W (GPB2) | YDR417C | YGR018C | YLR292C (SEC72) |
| YAR003W (SWD1) | YDR442W | YGR025W | YLR295C (ATP14) |
| YBL099W (ATP1) | YDR525W-A (SNA2) | YIL073C (SPO22) | YLR333C (RPS25B) |
| YBR016W | YER083C (GET2) | YIL146C (ECM37) | YLR454W (FMP27) |
| YBR068C (BAP2) | YER185W (PUG1) | YIL159W (BNR1) | YML121W (GTR1) |
| YBR073W (RDH54) | YFL043C | YIR003W (AIM21) | YNR049C (MSO1) |
| YBR089C-A NHP6B) | YFR008W (FAR7) | YJR009C (TDH2) | YOL023W (IFM1) |
| YBR093C (PHO5) | YGL058W (RAD6) | YJR010C-A (SPC1) | YOR147W (MDM32) |
| YBR147W (RTC2) | YGL064C (MRH4) | YLL026W (HSP104) | YOR298C-A (MBF1) |
| YBR156C (SLI15) | YGL127C (SOH1) | YLL046C (RNP1) | YPL183C (RTT10) |
| YCL028W (RNQ1) | YGL203C (KEX1) | YLL053C | |
| YDL100C (GET3) | YGL217C | YLR003C (CMS1) |
Table 3.
Gene deletions that contain a mixed population of [PIN+] and [pin−] cells
| YBL107C | YDR491C | YIL041W (GVP36) | YMR307W (GAS1) |
| YBR001C (NTH2) | YDR506C | YIL073C (SPO22) | YNL011C |
| YBR010W (HHT1) | YDR552C (SPS2) | YJL003W (COX16) | YNL109W |
| YBR044C (TCM62) | YER141W (COX15) | YJL007C | YNL141W (AAH1) |
| YBR114W (RAD16) | YFL033C (RIM15) | YJR020W | YNL187W (SWT21) |
| YBR159W (IFA38) | YFL044C (OTU1) | YJR024C (MDE1) | YNL199C (GCR2) |
| YBR212W (NGR1) | YFL047W (RGD2) | YJR051W (OSM1) | YNL230C (ELA1) |
| YCR022C | YFL049W (SWP82) | YJR053W (BFA1) | YNL276C |
| YCR034W (FEN1) | YFL054C | YLF046W (FMP32) | YOL023W (IFM1) |
| YDL011C | YFR006W | YLF048C (EMP47) | YOL152W (FRE7) |
| YDL090C (RAM1) | YFR015C (GSY1) | YLR263W (RED1) | YOR138C (RUP1) |
| YDL134C (PPH21) | YFR020W | YLR292C (SEC72) | YOR161W (LIP5) |
| YDR260C (SWM1) | YGL109W | YLR304C (ACO1) | YOR274W (MOD5) |
| YDR283C (GCN2) | YGL127C (SOH1) | YLR331C (JIP3) | YOR379C |
| YDR286C | YGL226W | YML061C (PIF1) | YPL148C (PPT2) |
| YDR287W (INM2) | YGL252C (RTG2) | YML121W (GTR1) | YPR054W (SMK1) |
| YDR294C (DPL1) | YGR007W (MUQ1) | YML129C (COX14) | YPR060C (ARO7) |
| YDR333C | YGR268C (HUA1) | YMR082C | YPR069C (SPE3) |
| YDR443C (SSN2) | YHL009C (YAP1) | YMR202W (ERG2) | YPR092W |
| YDR459C (PFA5) | YHR038W (RRF1) | YMR247C (RKR1) | YPR093C (ASR1) |
| YDR481C (PHO8) | YHR090C (YNG2) | YMR282C (AEP2) |
[PIN+] variants are maintained in candidate strains
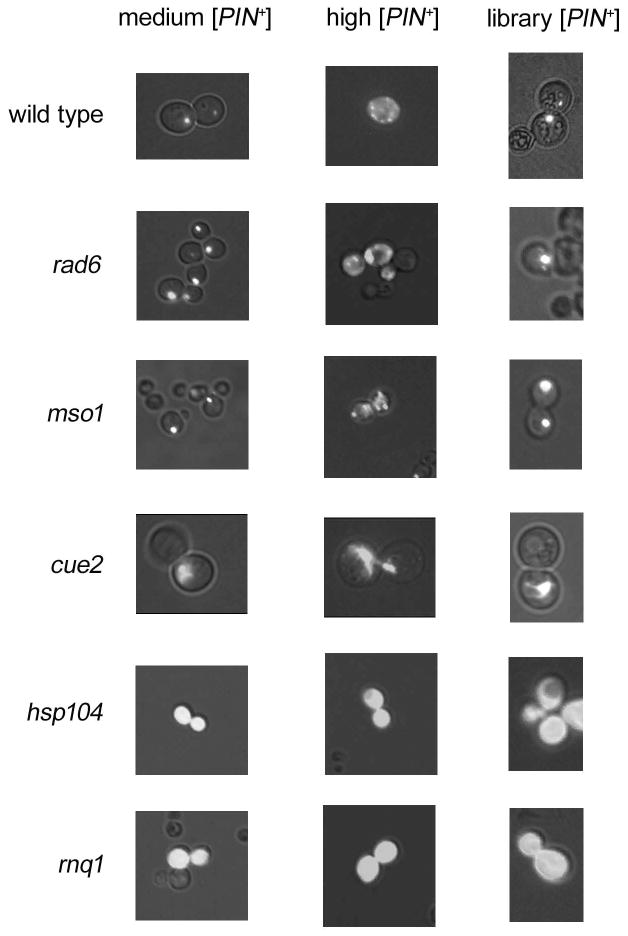
We next asked if any of the deletions in the 141 candidate library strains caused the loss of the [PIN+] prion, or if these strains had become [pin−] by chance. As described above, [PIN+] variants exhibit different phenotypes and stabilities. Therefore, we tested whether the propagation of other [PIN+] variants was affected in the candidate deletion strains. Upon overexpression of RNQ1:GFP, medium [PIN+] containing cells have single foci, similar to the variant found in the library, whereas a high [PIN+] cell contains numerous smaller foci (Bradley and Liebman, 2004). Introduction of these variants into candidate strains showed a consistent profile. For 138 strains, medium [PIN+] exhibited stable single-dot aggregation profiles, whereas high [PIN+] displayed stable multiple-dot profiles (Fig. 2). It appears that these [PIN+] variants are maintained after extended growth, since the majority of cells selected on the first pass of Gal-Lys-Leu media (see materials and methods) are cytoductants, and these cytoductants are propagated on various media over a two-week span (Fig. 1). While [PIN+] instability would yield a mixed population of diffuse and aggregated cells, no diffuse cells were detected in the 138 candidate strains. Furthermore, storage of strains at 4°C for several days did not appear to affect [PIN+] instability. Only hsp104Δ and rnq1Δ strains showed diffuse fluorescence (Fig. 2).
Figure 2. All candidate strains, except for hsp104Δ and rnq1Δ, maintained three variants of [PIN+].
Medium [PIN+] (left), high [PIN+] (middle) or the library [PIN+] (right) was cytoduced into each candidate strain, along with the RNQ1:GFP inducible plasmid. Strains were induced for 16 hours with copper. Wild type strains (trp1Δ) show punctate fluorescence with all variants. rad6Δ and mso1Δ are representative phenotypes of 138 candidate strains that maintained [PIN+]. A library strain (YKL090w) containing a deletion of the cue2 gene shows spider-like fluorescence regardless of the [PIN+] variant.
The strain containing a deletion of the gene encoding the monoubiquitin binding protein, Cue2p (Kang et al., 2003) displayed bright large spider-like RNQ1:GFP fluorescence, regardless of the [PIN+] variant introduced (Fig. 2), but the phenotype was less extreme when the deletion was re-engineered in a different strains background (74-D694; data not shown). Analysis of large molecular weight aggregates analyzed by sucrose gradient, as well as small SDS-resistant Rnq1p oligomers detected by SDD-AGE (Bagriantsev and Liebman, 2004) showed no differences in the state of Rnq1p aggregation between cue2Δ and wildtype strains (data not shown)
We found that kar1 plasmid donor strains containing either low or very high variants of [PIN+], but not medium or high [PIN+], lost the prion in a percentage of the population when left at 4°C for over two months (data not shown). Since medium and high [PIN+] have less soluble Rnq1p, they may be more stable. The properties of the [PIN+] variant in the library are unknown. Therefore, it was important to test if this library variant of [PIN+] was also maintained by all candidate strains. Thus, the library version of [PIN+] was reintroduced into the 141 candidate strains using the kar1 plasmid donor. 138 strains contained aggregates after cytoduction and therefore were able to maintain the newly introduced [PIN+] (Fig 2). In addition, these deletion strains were also able to maintain the [PSI+] prion (data not shown). Again, only hsp104Δ and rnq1Δ strains showed diffuse fluorescence, and the cue2Δ strain showed an altered aggregation phenotype similar to above (Fig 2). These results indicate that HSP104 and RNQ1 are the only two non-essential genes required for the maintenance of the [PIN+] prion.
Discussion
This study determined which of the 5155 strains within the yeast deletion library contain the [PIN+] prion and that less than 3% of the library strains contain [pin−] cells. Furthermore, we showed that all the [pin−] library strains, except for hsp104Δ and rnq1Δ, could maintain three different [PIN+] variants. This suggests that the 139 other strains that lost [PIN+] in the library did so by chance and not because of the deletions they carry.
The presence of [PIN+] in the library can alter the outcome of genetic screens. For example, [PIN+] has been shown to enhance toxicity of the expanded poly-glutamine fragment associated with Huntington’s disease in yeast (Meriin et al., 2002). While a screen of the yeast deletion library identified 28 suppressors of Huntington-associated toxicity (Giorgini et al., 2005), our study reveals that 18 of these candidates did not display toxic effects because they were most likely [pin−].
Additionally, we found that a deletion of the CUE2 gene does not affect prion propagation but curiously alters the aggregation of RNQ1:GFP in [PIN+] cells. A re-engineered version of cue2Δ showed a less drastic phenotype and biochemical profiles were similar to wildtype strains, thus, we did not pursue further characterization.
While HSP104 and RNQ1 are the only non-essential genes that affect the maintenance of [PIN+], elevating the protein levels of certain proteins (Chernoff et al., 1995; Yang, Hong and Liebman, unpublished) or affecting the function of multiple non-essential genes (Jung et al., 2000) have been shown to greatly affect prion maintenance. Essential genes also could be crucial for prion propagation: e.g. SIS1, an essential Hsp40 member, is required for faithful propagation of [PIN+] and [PSI+] (Sondheimer et al., 2001; Higurashi et al., 2008). Furthermore, it was shown that the presence of [PIN+] in a sla2Δ GT81 (Ganusova et al., 2006) strain leads to extremely slow growth (Ganusova et al., 2006), and we find the same toxic effect in a sla2Δ [PIN+] BY4741 strain (Manogaran and Liebman, unpublished), which is not included in the library. While strains in the original release and two subsequent updates of the library (tested in this study) are not associated with [PIN+] toxicity, there are a handful of non-essential genes that are not included in these collections. Investigation of these remaining non-essential genes could reveal other factors that play a role in prion propagation.
Acknowledgments
This work was supported by the U.S. Army (48145LS to S.W.L.), the National Institutes of Health NSRA F32 postdoctoral fellowship (GM072340 to A.L.M), the National Institutes of Health grants (CA125520, GM50237, and HG0237 to R.R., and HG00193 to R.J.D.R), and the University of Illinois at Chicago Hispanic Center of Excellence (to V.M.F.).
References
- Bagriantsev S, Liebman SW. Specificity of Prion Assembly in vivo: [PSI] and [PIN] form separate structures in yeast. J Biol Chem. 2004;279:51042–51048. doi: 10.1074/jbc.M410611200. [DOI] [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′ phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bradley ME, Edskes HK, Hong JY, Wickner RB, Liebman SW. Interactions among prions and prion “strains” in yeast. Proc Natl Acad Sci USA. 2002;99:16392–16399. doi: 10.1073/pnas.152330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ME, Liebman SW. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol Micro. 2004;51:1649–1659. doi: 10.1111/j.1365-2958.2003.03955.x. [DOI] [PubMed] [Google Scholar]
- Bruce ME. TSE strain variation. British Medical Bulletin. 2003;66:99–108. doi: 10.1093/bmb/66.1.99. [DOI] [PubMed] [Google Scholar]
- Chlebowicz-Sledziewska E, Sledziewski AZ. Construction of multicopy yeast plasmids with regulated centromere function. Gene. 1985;39:25–31. doi: 10.1016/0378-1119(85)90103-9. [DOI] [PubMed] [Google Scholar]
- Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci USA. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147:507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: the story of [PIN+] Cell. 2001;93:171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- Diaz-Avalos R, King CY, Wall J, Simon M, Caspar DL. Strain-specific morphologies of yeast prion amyloid fibrils. Proc Natl Acad Sci USA. 2005;102:10165–10170. doi: 10.1073/pnas.0504599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Mol Cell Biol. 2006;26:617–629. doi: 10.1128/MCB.26.2.617-629.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva B, Rothstein R. Kar-mediated plasmid transfer between yeast strains: an alternative to traditional transformation methods. Methods Enzymol. 2002;350:278–89. doi: 10.1016/s0076-6879(02)50969-1. [DOI] [PubMed] [Google Scholar]
- Giaever G, Flaherty P, Kumm J, Proctor M, Nislow C, Jaramillo DF, Chu AM, Jordan MI, Arkin AP, Davis RW. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc Natl Acad Sci USA. 2004;101:793–798. doi: 10.1073/pnas.0307490100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen QV, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington’s disease. Nature Genetics. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi T, Hines JK, Sahi C, Aron R, Craig EA. Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc Natl, Acad, Sci, USA. 2008;105:16596–16601. doi: 10.1073/pnas.0808934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, Proctor M, St Onge RP, Tyers M, Koller D, Altman RB, Davis RW, Nislow C, Giaever G. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008;320:362–365. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Jones G, Wegrzyn RD, Masison DC. A role for cytosolic hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics. 2000;156:559–70. doi: 10.1093/genetics/156.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Masison DC. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prion. Curr Microbiol. 2001;43:7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- Kang RS, Daniels DM, Francis SA, Shih SC, Salerno WJ, Hicke L, Radhakrishnan I. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell. 2003;113:621–630. doi: 10.1016/s0092-8674(03)00362-3. [DOI] [PubMed] [Google Scholar]
- King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Lindquist SL. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature. 2005;435:765–772. doi: 10.1038/nature03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem. 2003;278:49636–49643. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Bio. 2002;157:997–1004. doi: 10.1083/jcb.200112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Sunjevaric I, Voth WP, Ciccone S, Du W, Olsen AE, Stillman DJ, Rothstein R. Chromosome-scale genetic mapping using a set of 16 conditionally stable Saccharomyces cerevisiae chromosomes. Genetics. 2008;180:1799–808. doi: 10.1534/genetics.108.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1990. [Google Scholar]
- Schlumpberger M, Prusiner SB, Herskowitz I. Induction of distinct [URE3] yeast prion strains. Mol Cell Biol. 2001;21:7035–7046. doi: 10.1128/MCB.21.20.7035-7046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1986. [Google Scholar]
- Sondheimer N, Lindquist SL. Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- Sondheimer N, Lopez N, Craig EA, Lindquist SL. The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 2001;20:2435–2442. doi: 10.1093/emboj/20.10.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- Tong AH, Evangelista M, Parsons AB, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- Tuite MF, Mundy CR, Cox BS. Agents that cause a high frequency of genetic change from the [psi+] to [psi−] in Saccharomyces cerevisiae. Genetics. 1981;98:691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Kelly MJ, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–237. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Madariaga ML, Lindquist S. Prion switching in response to environmental stress. PLoS Biol. 2008;6:e294. doi: 10.1371/journal.pbio.0060294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]