Abstract
Methamphetamine interferes with dopamine reuptake, and the resulting increased dopamine oxidation that creates oxidative stress can lead to degeneration of dopaminergic terminals. Previous studies have shown that the trace element selenium protects against methamphetamine toxicity. However, the specific selenoproteins responsible for protection have not been elucidated. Glutathione peroxidases 1 and 4 (GPx1 and GPx4) incorporate selenium into the amino acid selenocysteine, and their known antioxidant functions make them good candidates for protection from methamphetamine-induced oxidative damage. We differentiated SH-SY5Y neuronal cells in serum-free media with defined supplement containing 0, 10 and 100 nM selenium, and then challenged the cells with a 24-hour exposure to methamphetamine. We found that 100 μM methamphetamine decreased GPx1 and GPx4 protein levels. However, both proteins were upregulated with increasing media selenium concentration. GPx enzymatic activity was also increased by selenium and decreased by methamphetamine and correlated with GPx protein levels. Total glutathione levels were reduced by methamphetamine at lower selenium conditions, while the oxidized fraction of GSH was increased at higher selenium levels. Additionally, we observed an increased generation of reactive oxygen species with methamphetamine exposure in media with 0 nM selenium, which was ameliorated by selenium supplementation. These results show that methamphetamine increases oxidative stress by reducing GPx levels, and this can be reversed with addition of selenium. These findings have important implications for treating patients with acute methamphetamine toxicity.
Keywords: Methamphetamine, selenium, glutathione peroxidase, glutathione, dopamine, oxidative stress
1. Introduction
Abuse of methamphetamine (Meth), a highly addictive drug with psychostimulant properties, is a rapidly growing health problem with millions of users worldwide (Krasnova and Cadet, 2009). Although initially increasing sense of pleasure and desire for social interaction, Meth use can increase depression and induce paranoia and aggression (Homer et al., 2008). Chronic abuse can lead to lasting neuronal damage (Ernst et al., 2000). Understanding Meth neurotoxicity and finding viable treatments are both important to ensure both individual recovery as well as decreasing the financial burden that the Meth epidemic imposes on society.
Meth increases release of dopamine (DA) onto striatal neurons (Seiden et al., 1993). Meth enters terminals though the dopamine transporter (DAT), and causes release of DA from synaptic vesicles by disrupting the proton gradient necessary for function of the vesicular monoamine transporter-2 (VMAT) (Sulzer et al., 1992, Sulzer and Rayport, 1990). Meth can increase oxidative stress at DA terminals and cause damage through a build-up of oxidized forms of DA and generation of free radicals though various pathways (Quinton and Yamamoto, 2006). Selenium (Se) deficiency causes an increase in DA turnover and expression of tyrosine hydroxylase (TH) (Castano et al., 1995, Castano et al., 1997, Romero-Ramos et al., 2000). Se supplementation has been shown to exert protective effects against Meth-induced toxicity in rodent and cell culture models. Animal studies also demonstrate that Meth-induced dopaminergic toxicity in striatum is potentiated by Se deficiency (Kim et al., 2000), and attenuated by Se supplementation (Imam et al., 1999a) through ameliorating oxidative stress (Imam et al., 2001, Kim et al., 1999a). Meth-induced dopaminergic toxicity and peroxynitrite generation in cultured cells is also alleviated by Se (Imam and Ali, 2000b).
Although studies have demonstrated protective effects of Se against Meth toxicity, little is known about the mechanism of Se protection. The functions of Se are carried out mainly by selenoproteins, in which Se is specifically incorporated as selenocysteine (Berry et al., 1993). However, the selenoproteins involved in protection against Meth have not been identified. Several selenoproteins are involved in redox reactions and have antioxidant properties. The glutathione peroxidase (GPx) selenoproteins utilize glutathione as a substrate to reduce peroxide and other free radicals, and thus are likely candidates for Meth protection.
In neuron cultures, Se in organic and inorganic forms can be found in fetal bovine serum (FBS), a typical ingredient of most culture media. The Se transporter selenoprotein P (Sepp1) was previously isolated from FBS as a factor promoting survival of neurons in culture (Yan and Barrett, 1998). However, inorganic Se in the form of sodium selenite can be used in media supplements in place of Sepp1 (Brewer et al., 1993). Manipulating Se content of culture media is impaired by the presence of Se in FBS and media supplements. However, a recently published media formulation similar to the popular but proprietary Gibco B27 supplement allows alteration of Se (Roth, Zhang, 2010).
We hypothesized that one or more selenoproteins are increased by Se supplementation and have protective properties against Meth. In this study, we examined the expression of glutathione peroxidases 1 and 4 (GPx1 and GPx4) in response to Meth and selenium supplementation. We report here that both GPx1 and GPx4 are increased by Se supplementation and reduced by Meth.
2. Materials and methods
2.1. Cell culture conditions
We prepared serum-free media using a media supplement recently described by Schweizer and colleagues (Roth, Zhang, 2010). This supplement is similar to the B27 supplement (Brewer, Torricelli, 1993) available commercially (Gibco), and is composed of the same reagents. These include: biotin, L-carnitine, ethanolamine, D(+)-galactose, putrescine dihydrochloride, albumin (bovine), catalase, glutathione, reduced superoxide dismutase, apo-transferrin, Na2SeO3, ZnSO4, CuSO4, MnCl2, NH4VO3, corticosterone, linoleic acid, linolenic acid, progesterone, retinyl acetate D, L-α-tocopherol D, L-α-tocopherol acetate, Lipoic acid, Insulin (human), and 3,3′,5-triiodo-L-thyronine (T3). All supplemental reagents were purchased from Sigma, and were added directly to Neurobasal media (Invitrogen). The sodium selenite (Na2SeO3) was added to the media last at 0, 10, or 100 nM concentrations for Se deplete, Se low, and Se supplemented conditions.
SH-SY5Y cells were plated in DMEM (Gibco) with 10% FBS (Gibco). After 24 hrs, the media was changed to Neurobasal Medium (Gibco) containing B27 supplement (Gibco), with the addition of glutamine (2mM), penicillin (1000U/ml), and streptomycin (100 μg/ml). Cells were grown in this media for two days, and retinoic acid in the B27 supplement formulation induced neuronal differentiation. For undifferentiated cell cultures, Schweizer supplement without retinoic acid was used in place of the B27 supplement.
Cells were differentiated in Neurobasal with Schweizer supplement containing varying concentrations of Se for two days for the dose response curve and two weeks for all other experiments. Longer differentiation periods for SH-SY5Y cells increase expression of tyrosine hydroxylase and result in a phenotype more closely resembling DA neurons (Lopes et al., 2010). Following differentiation, media was changed to that containing Se and methamphetamine hydrochloride (100 μM) 24 hrs prior to harvesting cells for protein.
2.2. Western blot analysis
Protein was harvested with CelLytic solution (Sigma) per manufacturer’s instructions. Samples were heated in Laemmli buffer to 95°C, run on 10–20% gradient Criterion Tris-HCl gels (Bio-Rad), and transferred by electrophoresis to PVDF membranes at 12V overnight. Membranes were incubated in blocking buffer (Odyssey) for 1 hr. Membranes were then incubated overnight at 4°C in primary antibody at dilutions below in 5 mL blocking buffer and 5 mL 0.01% Tween20 (Sigma) 1X PBS (PBST) followed by three 10 min washes with 1X PBST. Next, membranes were incubated with secondary antibody diluted 1:10,000 in 1X PBST for 30 min followed by three 10-min washes with 1X PBST. Proteins were identified with 1:2000 goat anti-GPx1 (GenWay), 1:2000 rabbit anti-GPx4 (AbFrontier), and 1:10,000 anti-alpha tubulin (Novus). Protein was imaged and analyzed using the Odyssey Infrared Imaging System (LI-CORE, Lincoln, NE). Separate western blots had samples from each condition, and samples on replicate blots were normalized to the 10 nM Se, 0 μM Meth sample within the same blot.
2.3 Determination of Meth toxicity
SH-SY5Y cells were plated in 96-well plates in Neurobasal with B27 supplement. After differentiation for >2 weeks, culture media was changed to that containing Meth at 0, 50 or 100 μM. Following 48-hr Meth exposure, cell viability was determined using CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS) (Promega) as per manufacturer’s instructions. A solution containing phenazine methosulfate (PMS) and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was added to the culture media and changes in absorbance at 490 nm were measured after 1 hr using a plate reader.
2.4. Determination of GPX activity by GPx-340 assay
SH-SY5Y cells were incubated with 0, 10, or 100 nM Se for one week followed by a 24-hr Meth treatment. Protein concentration was determined by Bradford assay. GPx activity was determined using BIOXYTECH GPx-340 assay kit (Oxis) per manufacturer’s instructions. Protein was harvested with CelLytic solution and mixed with 25 μL assay buffer, 25 μL NADPH, and 25 μL 1:10,000 tert-Butyl Hydroperoxide. The decrease in absorption at 340 nm was monitored every 2 sec for 5 min. The rate of decrease is directly proportional to GPx activity. Results are displayed as GPx activity/protein concentration.
2.5. Determination of GSH and GSSG by GSH/GSSG-412 assay
Glutathione (GSH) and oxidized glutathione (GSSG) concentration were determined using Oxis GSH/GSSG-412. SH-SY5Y cells were treated with 0, 10, or 100 nM Se for one week followed by a 24-hr Meth treatment. Protein was harvested with proteinase inhibitor cocktail set III (Calbiochem) diluted in 1X PBS and dissociated by sonication. An aliquot of protein extract was mixed with 1-methyl-vinul-pyridium trifluoromethane sulfonate (M2VP) in HCl (Oxis) to be used in determining GSSG concentration. Protein extract and protein aliquot containing M2VP were mixed with 20 μL of 1X Glutathione reductase (Sigma), 20 μL Chromagen (Oxis), 17 μL assay buffer and 20 μL of 0.25 mM NADPH (Oxis). The increase in absorbance at 412 nm wavelength was monitored every 12 sec for 5 min. Results were transformed to total GSH (tGSH) and GSSG concentrations, calculated by comparing to the standard curve provided by the kit. Results are displayed as GSH concentration/protein concentration. Protein concentration was determined by ND-1000 Nanodrop spectrophotometer (Thermo Scientific).
2.6 Determination of oxidative stress
Oxidative stress was measured using a 2′,7′dichlorofluorescein diacetate (DCFH-DA) fluorescent assay. SH-SY5Y cells were treated with 0, 10, or 100 nM Se for one week followed by a 24-hr Meth treatment. Protein extract was added to 100 μL of 5μM DCFH-DA dissolved in serum-free media supplement. The increase in fluorescence was monitored every 2 min for 1 hr at 485 nm excitation and 535 nm emission. Results were transformed to amount (nmol) of product/min/mg protein using a standard curve generated with the following concentrations of DCF dissolved in Neurobasal with Schweizer supplement: 10 nM, 50 nM, 100 nM, 500 nM, 1μM, 5 μM, 10 μM.
2.7. Statistical analysis
Experimental groups are expressed as means ± SEM. Differences between samples with one variable were analyzed using 1-way ANOVA with Bonferoni’s posthoc test, and differences between group means in experiments with more than one variable were compared using 2-way ANOVA with Bonferoni’s posthoc test. P values < 0.05 are considered statistically significant. Relationships between variables were determined using linear regression analysis.
3. Results
3.1 GPx Protein
We investigated the levels of two antioxidant selenoproteins, GPx1 and GPx4, in differentiated SH-SY5Y cells in response to Meth treatment for 24 hrs. As shown in Fig. 1A, GPx1 protein was unchanged in undifferentiated cells. However, the concentration of GPx1 protein decreased with increasing concentrations of Meth (right). There was a significant reduction in protein concentration at 100 μM Meth. We found a similar protein expression pattern with GPx4. While GPx4 levels did not change in undifferentiated cells, GPx4 was decreased in differentiated cells (Fig. 1B). We also measured the Meth toxicity in differentiated cells using an MTS cell proliferation assay. Cell viability decreased with 50 and 100 μM Meth concentrations (Fig. 1C), corresponding to the decrease in GPx levels.
Figure 1.
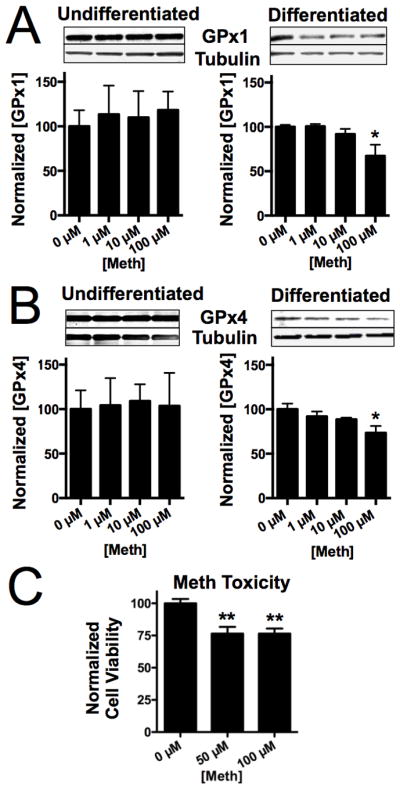
Meth decreases GPx protein in a concentration-dependent manner. A. In undifferentiated cells (left), GPx1 levels did not change with Meth. However, in differentiated cells (right), increasing Meth results in decreased GPx1 protein expression whereas in undifferentiated cells, GPx1 protein expression remained unchanged. Above blot shows representatives of three samples for each Meth concentration. B. Meth also decreases GPx4 in differentiated cells (right) but unchanged in undifferentiated cells (left). C. Meth (50 and 100 μM) decreased cell viability after 48 hrs measured with an MTS cell proliferation assay. Bars show mean ± SEM for three replicate cultures per condition. *indicates p<0.05, ** indicates p<0.01(One-Way ANOVA with Bonferoni’s posthoc test).
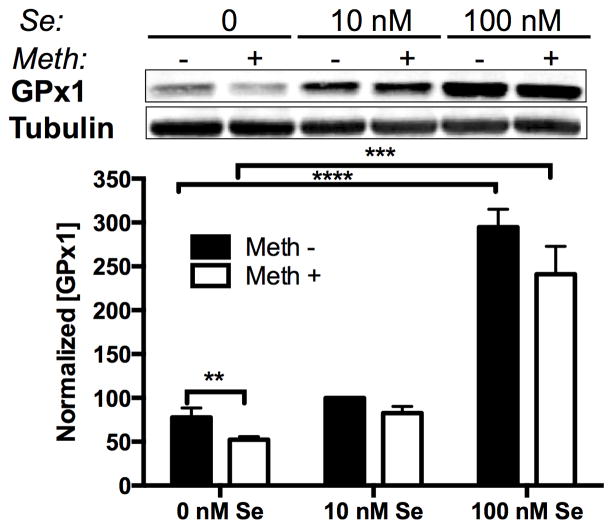
We next considered if Se levels in media affected the response of GPx protein levels to Meth. As the GPx enzymes are selenoproteins that incorporate Se into the amino acid selenocysteine (Sec), we hypothesized that selenoprotein synthesis may be limited by the availability of Se. In Fig. 2, we observed a modest increase in GPx1 protein from 0 nM to 10 nM, and a further large increase from 10 nM to 100 nM media. There was an overall significant effect of Se on GPx1 protein level. In 0 nM Se conditions, Meth (100 μM) decreased GPx1 protein levels compared to untreated cells. Although there appeared to be no interaction between Se and Meth, 10 nM Se and 100 nM Se levels increased GPx1 levels to the point where Meth-induced reduction was inconsequential.
Figure 2.
Methamphetamine and selenium alter GPx1 protein levels. Above: Representative western blot of GPx1 from SH-SY5Y cells grown in media containing 0, 10, or 100 nM Se, with or without 100 μM Meth. Four replicate cultures for each condition were measured. Below: Graph of GPx1 protein mean ± SEM measured by optical density of western blot bands. Bars show mean ± SEM for four replicate cultures per condition. ** indicates p<0.01, *** indicates p<0.001, and **** indicates p<0.0001 (Two-Way ANOVA with Bonferoni’s posthoc test).
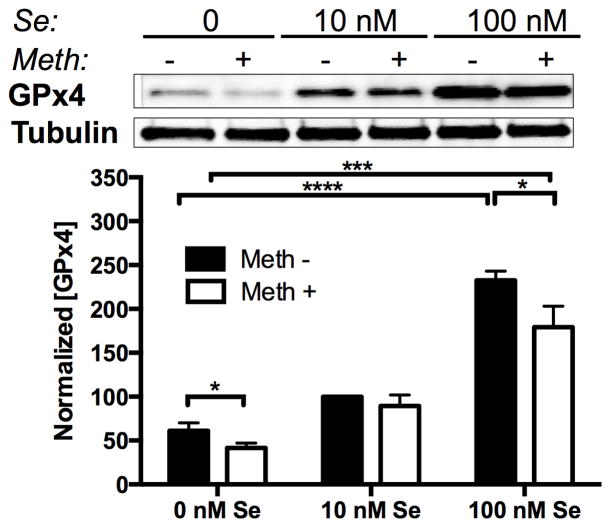
We also measured GPx4 levels in response to Se and Meth (Fig. 3). As with GPx1, GPx4 protein levels increased with increasing Se and decreased following exposure to 100 μM Meth. At 0 nM Se, GPx4 in Meth treated-cultures was significantly reduced compared to non-Meth controls. Although Meth reduced GPx4 at 100 nM Se, GPx4 at the highest Se concentration co-treated with Meth was much greater then that at lower Se concentrations without Meth.
Figure 3.
Methamphetamine and selenium alter GPx4 protein levels. Above: Representative western blot of GPx4 from SH-SY5Y cells grown in 0, 10, or 100 nM Se, with or without 100 μM Meth. Bars show mean ± SEM for four replicate cultures per condition. Below: Graph of GPx4 protein mean ± SEM measured by optical density of western blot bands. * indicates p<0.05, *** indicates p<0.001, and **** indicates p<0.0001 (Two-Way ANOVA with Bonferoni’s posthoc test).
3.2 Glutathione levels
Changes in GPx activity in response to Se and Meth (Fig. 4A) were very similar to changes in protein levels. Increased Se concentration in the media resulted in increased GPx activity. However, the presence of 100 μM Meth decreased GPx activity. GPx activity was lowest in 0 nM Se media with 100 μM Meth. The similarity of GPx activity with GPx protein suggests that reduced activity is likely due to lowered protein concentrations. To test this, we examined the correlation of GPx1 and GPx4 protein with GPx activity using linear regression. There was a strong positive correlation between GPx activity GPx1 (Fig. 4B) and GPx4 (Fig. 4C) protein levels in cells from media with and without Meth.
Figure 4.
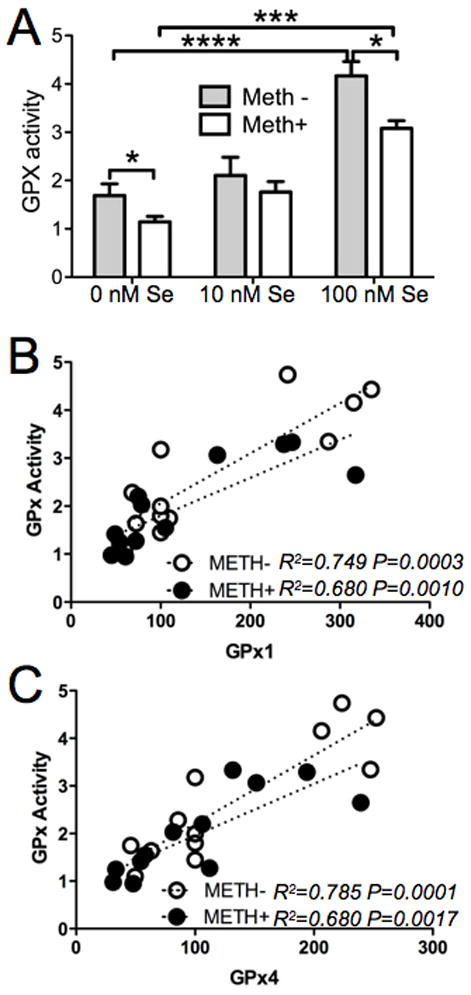
Meth decreases GPx enzymatic activity. A. GPx enzyme activity was measured from SH-SY5Y cell lysates from cells grown in 0, 10 or 100 nM Se, with or without 100 μM Meth. Bars show mean ± SEM for four replicate cultures per condition. * indicates p<0.05, *** indicates p<0.001, and **** indicates p<0.0001 (Two-Way ANOVA with Bonferoni’s posthoc test). B. Linear correlation between GPx activity and GPx1 protein levels. C. Linear correlation between GPx activity and GPx4 levels.
To further characterize GPx function, we measured the intracellular content of GSH, which is a co-factor for GPx enzymes. As shown in Figure 5A, Meth treatment decreased the tGSH concentration in cells in 0 nM and 10 nM Se conditions, but not at supplemented Se levels. Without Meth treatment, the tGSH concentration was higher in 0 nM and 10 nM Se media, and decreased with Se supplementation. As shown in Fig. 5B, the oxidized GSH fraction ([GSSG]/[tGSH] ratio) was reduced in 10 nM Se media compared to 0 nM Se. In 10 nM and 100 nM Se media, the addition of Meth significantly increased the [GSSG]/[tGSH] ratio. Meth exposure increased GSSG when Se was present in the media, but did not change the redox status of GSH in Se-deficient cells (0 nM Se). Overall, the addition of Meth either decreased total GSH in the absence of Se or increased the GSSG fraction in the presence of Se.
Figure 5.
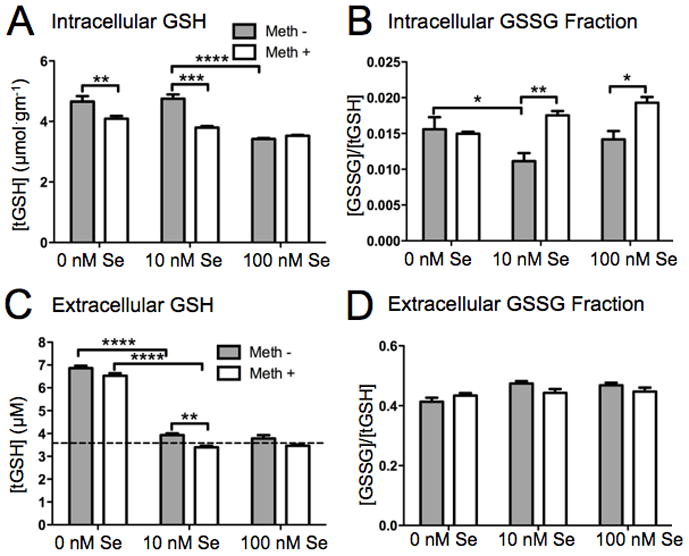
Meth- and Se-induced changes in total GSH (tGSH) content and oxidized GSH (GSSG) fraction. tGSH and GSSG were measured in lysates and media from SH-SY5Y cells differentiated in media containing 10 nM Se for 2 weeks followed by either a 0, 10, or 100 nM Se for 1 week, and then treated with or without 100 μM Meth for 24 hrs. A. Intracellular tGSH is decreased at 100 nM relative to 0 and 10 nM Se without Meth. Addition of 100 μM Meth decreases tGSH at 0 and 10 nM Se. B. The ratio of intracellular GSSG to tGSH is increased by addition of 100 μM Meth at 10 nM and 100 nm Se. Two-way ANOVA and Bonferroni post hoc test were used. Samples from three replicate cultures were used, with four assays averaged for each sample. C. Extracellular tGSH is increased in media containing 0 nM Se. Horizontal line indicates the amount of GSH originally added to the media (3.6 μM). D. The ratio of extracellular GSSG to tGSH in unchanged by Se content of media or addition of Meth. Bars show mean ± SEM for four replicate cultures per condition. Total **** indicates p<0.0001, *** indicates p<0.001, ** indicates p<0.01, * indicates p<0.05.
Because glutathione is a component of the extracellular media (Roth, Zhang, 2010), we also measured GSH and GSSG in the media taken from cells when harvested. We found that tGSH was significantly increased in 0 nM Se media relative to 10 nM Se media, independent of Meth treatment (Fig 5C). In media with 10 nM and 100 nM Se, the measured tGSH was close to the concentration of glutathione added to the media (3.6 μM – represented by horizontal dashed line in Fig. 5C). This indicates that GSH is actually released from cells grown in Se-deplete media. The tGSH in 10 nM Se media was significantly lower when Meth was added, apparently due to a slight increase in extracellular tGSH in the absence of Meth. Thus a smaller amount of GSH released into the media in 10 nM Se compared to 0 nM Se is further reduced by Meth, possibly due to greater utilization of intracellular tGSH. The ratio of extracellular GSSG to tGSH was not altered by Se or Meth (Fig. 5D).
3.3 ROS generation
We measured ROS levels in response to Se and Meth (Fig. 6). At 0 nM Se there was a significant increase in ROS in the presence of 100 μM Meth. However, there was no increase in ROS in cells treated with Meth co-treated with 10 nM and 100 nM Se. This is in agreement with the GPx protein and activity levels, as increased GPx due to the presence of high Se would prevent ROS increases from Meth.
Figure 6.
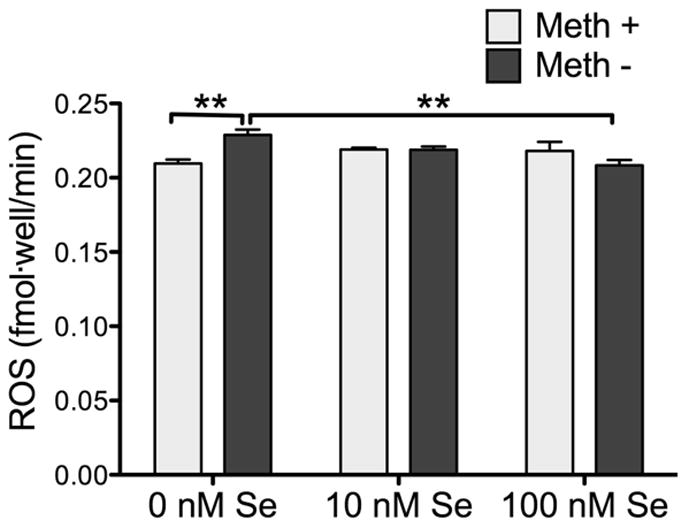
Meth-induced generation of ROS in SH-SY5Y cells in 0 nM Se. ROS was measured by DCFH assay. Bars show mean ± SEM for four replicate cultures per condition. ** indicates p<0.01, (Two-Way ANOVA with Bonferoni’s posthoc test, n = 4 cultures per condition)
4. Discussion
In this study, we show that exposure of SH-SY5Y cells to 100 μM Meth for 24 hrs can decrease GPx1 and GPx4 protein, as well as GPx enzymatic activity. Meth did not decrease GPx levels in undifferentiated cells, suggesting that synthesis of DA may be necessary for the decrease. The addition of Se to the media increases the GPx1 and GPx4 proteins levels, thus increasing cellular GPx activity. As shown in previous studies, expression of the GPx selenoenzymes increased with increasing Se (Lei et al., 1995, Weiss Sachdev and Sunde, 2001). Meth decreased tGSH and increased the ratio of GSSG to tGSH. We also found that Meth can increase ROS production at 0 nM Se, but not in the presence of 10 nM or 100 nM Se. These findings suggest that Se supplementation limits Meth-induced ROS production and prevents oxidative stress from Meth by transferring the oxidation to glutathione. Although previous studies have demonstrated that Se is protective against Meth toxicity (Imam and Ali, 2000a, Imam et al., 1999b, Kim et al., 1999b), the interaction of Se with Meth has been unclear. Our study shows the importance of Se to maintain levels of GPx enzymes as well as proper regulation of glutathione in order to reduce Meth-induced oxidative stress and improve protect against Meth toxicity.
Meth can increase oxidative stress within dopaminergic terminals (Yamamoto and Raudensky, 2008). DA is subject to auto-oxidation and enzymatic degradation, which results in the formation of hydrogen peroxide (H2O2) and superoxide (O2−) (LaVoie and Hastings, 1999). Meth also increases the extracellular concentration of glutamate (Nash and Yamamoto, 1992), which activates nitric oxide synthase and increases the production of nitric oxide (NO) (Dawson et al., 1992) and subsequently peroxynitrite (ONOO−). This leads to the inhibition of the mitochondrial electron transport chain (Bolaños et al., 1997, Moncada and Bolaños, 2006) at the site of complex II (Brown et al., 2005), which in turn increases the production of O2−. Rodent models have demonstrated that Meth administration increased lipid peroxidation, 3-Nitrotyrosine formation, and the production of hydroxyl radicals in the striatum (Imam, el-Yazal, 2001, Yamamoto and Zhu, 1998). The effectiveness of N-acetylcysteine amide in decreasing methamphetamine toxicity indicates that antioxidants may be a viable strategy (Zhang et al., 2012).
The GPx proteins are important for neuronal survival and function. The GPx family has long been known for reducing H2O2 and phospholipid hydroperoxides at the expense of glutathione (GSH) (Brigelius-Flohé, 2006). Interestingly, this enzyme family can also scavenge peroxynitrite, and the presence of GSH accelerates the reaction rate and sustains the enzyme activity (Briviba et al., 1998, Sies et al., 1997). Increased expression of GPx1 in PC-12 cells has previously been shown to protect against methamphetamine toxicity (Hom et al., 1997). In our study we found that both GPx1 and GPx4 proteins may contribute to the overall GPx activity during Meth exposure to reduce oxidative stress and potentially protect against Meth toxicity.
Further studies are needed to determine if the decreases in GPx proteins with Meth are due to decreased expression or increased protein degradation. It seems unlikely that cells would decrease the expression of a major antioxidant protein as a reaction to oxidative stress, although Meth may indirectly affect cell-signaling pathways to produce this outcome. The changes in GSH indicate increased activity of GPx proteins, which may increase the rate of enzyme degradation from entropy and eventual loss of protein through degradation pathways. The oxidized fraction of GSH increases with addition of Meth only in the presence of Se. This probably indicates that ROS increased by Meth is being reduced by GSH, which is partially mediated by GPx. Thus in 0 nM Se media, the decrease in GPx protein disrupts reduction of ROS by GSH. While an increased ratio of GSSG to tGSH is often used as an indicator of oxidative stress (Asensi et al., 1999), in our study the ratio increase reflects dependence upon GPx activity and indicates functional antioxidant activity. Total intracellular GSH was also lower in Se-supplemented conditions, suggesting competing mechanisms in GSH regulation. For example, supplementation with Se may reduce overall oxidation levels and also reduce GSH production by a negative feedback mechanism. Regardless, the addition of Meth decreased intracellular tGSH and increased the ratio of GSSG to tGSH, agreeing with reports of reduced tGSH from Meth exposure in animal models (Acikgoz et al., 2001, Mirecki et al., 2004).
We recently demonstrated that GPx4 is associated with dystrophic axons in Parkinson’s disease (PD), and may have a role in synthesis of neuromelanin (Bellinger et al., 2011). GPx4 prevents the buildup of oxidized lipids, which are metabolized by 12/15-lipoxygenase to produce products that activate apoptosis-inducing factor (AIF) to promote apoptotic cell death (Seiler et al., 2008). In the present study, we found that Meth exposure results in reduced GPx4 protein levels, which could result in an increase in oxidized lipids. However, the presence of Se in the media increases GPx4 protein to prevent the oxidation of lipids. Thus we were only able to detect an increase in ROS generation from addition of Meth in 0 nM Se media, as shown in Fig. 6.
5. Conclusion
These studies demonstrate the importance of dietary Se in prevention of Meth – induced oxidative stress. The reported poor nutrition among Meth users could thus exacerbate potential neurological damage (Morio et al., 2008). These findings suggest that treatment of acute Meth toxicity could be more effective if Se levels are tested and restored to adequate levels by Se supplementation. A better understanding of how Meth can alter the function of antioxidant proteins is warranted considering the growing worldwide increase in Meth use.
Highlights.
Methamphetamine decreased GPx1 and GPx4 enzymes in SH-SY5Y neuronal cells.
GPx1 and GPx4 increased with increasing culture media selenium concentrations.
Methamphetamine induced changes in glutathione levels and oxidized glutathione.
Glutathione changes were dependent upon selenium concentrations.
Methamphetamine increased reactive oxygen species in low but not high selenium.
Acknowledgments
The authors thank Linda Chang and Marla Berry for feedback and suggestions and Rachel Rueli for expert technical assistance. Supported by NIH R24 DA027318, as well as G12 MD007601 which supports the JABSOM histology and imaging core facilities.
Footnotes
Conflict of Interest Statement
The authors declare that they have no competing interests or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acikgoz O, Gonenc S, Gezer S, Kayatekin BM, Uysal N, Semin I, et al. Methamphetamine causes depletion of glutathione and an increase in oxidized glutathione in the rat striatum and prefrontal cortex. Neurotox Res. 2001;3:277–80. doi: 10.1007/BF03033266. [DOI] [PubMed] [Google Scholar]
- Asensi M, Sastre J, Pallardo FV, Lloret A, Lehner M, Garcia-de-la Asuncion J, et al. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–76. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bellinger MT, Seale LA, Takemoto AS, Raman AV, Miki T, et al. Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain. Mol Neurodegener. 2011;6:8. doi: 10.1186/1750-1326-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Banu L, Harney JW, Larsen PR. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993;12:3315–22. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, et al. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem. 1997;68:2227–40. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–35. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- Briviba K, Kissner R, Koppenol WH, Sies H. Kinetic study of the reaction of glutathione peroxidase with peroxynitrite. Chem Res Toxicol. 1998;11:1398–401. doi: 10.1021/tx980086y. [DOI] [PubMed] [Google Scholar]
- Brown JM, Quinton MS, Yamamoto BK. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. Journal of neurochemistr. 2005;95:429–36. doi: 10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Ayala A, Rodriguez-Gomez JA, de la Cruz CP, Revilla E, Cano J, et al. Increase in dopamine turnover and tyrosine hydroxylase enzyme in hippocampus of rats fed on low selenium diet. J Neurosci Res. 1995;42:684–91. doi: 10.1002/jnr.490420511. [DOI] [PubMed] [Google Scholar]
- Castano A, Ayala A, Rodriguez-Gomez JA, Herrera AJ, Cano J, Machado A. Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem Int. 1997;30:549–55. doi: 10.1016/s0197-0186(96)00123-4. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in the brain: The free radical, nitric oxide. Annals Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Hom DG, Jiang D, Hong EJ, Mo JQ, Andersen JK. Elevated expression of glutathione peroxidase in PC12 cells results in protection against methamphetamine but not MPTP toxicity. Brain Res Mol Brain Res. 1997;46:154–60. doi: 10.1016/s0169-328x(96)00296-3. [DOI] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychological bulletin. 2008;134:301–10. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res. 2000a;855:186–91. doi: 10.1016/s0006-8993(99)02249-0. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res. 2000b;855:186–91. doi: 10.1016/s0006-8993(99)02249-0. [DOI] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, et al. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci. 2001;939:366–80. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Islam F, Slikker W, Ali SF. Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res. 1999a;818:575–8. doi: 10.1016/s0006-8993(98)01311-0. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Islam F, Slikker W, Jr, Ali SF. Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res. 1999b;818:575–8. doi: 10.1016/s0006-8993(98)01311-0. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Choi DY, Im DH, Shin EJ, Suh JH, et al. Protection of methamphetamine nigrostriatal toxicity by dietary selenium. Brain Res. 1999a;851:76–86. doi: 10.1016/s0006-8993(99)02122-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Jhoo W, Shin E, Bing G. Selenium deficiency potentiates methamphetamine-induced nigral neuronal loss; comparison with MPTP model. Brain Res. 2000;862:247–52. doi: 10.1016/s0006-8993(00)02085-0. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Choi DY, Im DH, Shin EJ, Suh JH, et al. Protection of methamphetamine nigrostriatal toxicity by dietary selenium. Brain Res. 1999b;851:76–86. doi: 10.1016/s0006-8993(99)02122-8. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–91. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XG, Evenson JK, Thompson KM, Sunde RA. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr. 1995;125:1438–46. doi: 10.1093/jn/125.6.1438. [DOI] [PubMed] [Google Scholar]
- Lopes FM, Schroder R, da Frota ML, Jr, Zanotto-Filho A, Muller CB, Pires AS, et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010;1337:85–94. doi: 10.1016/j.brainres.2010.03.102. [DOI] [PubMed] [Google Scholar]
- Mirecki A, Fitzmaurice P, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, et al. Brain antioxidant systems in human methamphetamine users. J Neurochem. 2004;89:1396–408. doi: 10.1111/j.1471-4159.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–89. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Morio KA, Marshall TA, Qian F, Morgan TA. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. J Am Dent Assoc. 2008;139:171–6. doi: 10.14219/jada.archive.2008.0133. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–43. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. Aaps J. 2006;8:E337–47. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramos M, Venero JL, Cano J, Machado A. Low selenium diet induces tyrosine hydroxylase enzyme in nigrostriatal system of the rat. Brain Res Mol Brain Res. 2000;84:7–16. doi: 10.1016/s0169-328x(00)00171-6. [DOI] [PubMed] [Google Scholar]
- Roth S, Zhang S, Chiu J, Wirth EK, Schweizer U. Development of a serum-free supplement for primary neuron culture reveals the interplay of selenium and vitamin E in neuronal survival. J Trace Elem Med Biol. 2010;24:130–7. doi: 10.1016/j.jtemb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent-and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Sies H, Sharov VS, Klotz LO, Briviba K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem. 1997;272:27812–7. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S. Weak base model of amphetamine action. Ann N Y Acad Sci. 1992;654:525–8. doi: 10.1111/j.1749-6632.1992.tb26020.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Weiss Sachdev S, Sunde RA. Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. Biochem J. 2001;357:851–8. doi: 10.1042/0264-6021:3570851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–17. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–14. [PubMed] [Google Scholar]
- Yan J, Barrett JN. Purification from bovine serum of a survival-promoting factor for cultured central neurons and its identification as selenoprotein-P. J Neurosci. 1998;18:8682–91. doi: 10.1523/JNEUROSCI.18-21-08682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tobwala S, Ercal N. N-acetylcysteine amide protects against methamphetamine-induced tissue damage in CD-1 mice. Human & experimental toxicology. 2012;31:931–44. doi: 10.1177/0960327112438287. [DOI] [PubMed] [Google Scholar]