Abstract
Lewis rats show increased anxiety-like behaviors and drug consumption compared with Sprague-Dawley rats. Prior work suggests norepinephrine (NE) signaling in the bed nucleus of the stria terminalis (BNST) could have a role in mediating these phenotypes. Here, we investigated NE content and dynamics in the ventral BNST (vBNST) using fast-scan cyclic voltammetry in these two rat strains. We found that NE release evoked by electrical stimulus and its subsequent uptake was dysregulated in the more anxious Lewis rats. Because addiction is a multifaceted disease influenced by both genetic and environmental factors, we hypothesized NE dynamics would vary in these strains after the induction of a physical dependence on morphine. Following naloxone-precipitated morphine withdrawal, NE release and uptake dynamics were not changed in Lewis rats but were significantly altered in Sprague-Dawley rats. The alterations in Sprague-Dawley rats were accompanied by an increase in anxiety-like behavior in those animals as measured with the elevated plus maze. These studies suggest novel mechanisms involved in the development of affective disorders, and highlight the noradrenergic system in the vBNST as a common substrate for the manifestation of pathological anxiety and addiction.
Keywords: Norepinephrine, bed nucleus of the stria terminalis, morphine, neuronal release, norepinephrine uptake
INTRODUCTION
In drug-dependent individuals, researchers have highlighted the development of a persistent ‘negative emotional state' when access to drugs is terminated (Koob, 2009). Substance abuse is often co-morbid with anxiety disorders and impacted by stressful life experiences (Hyman et al, 2009; Sinha, 2008). The risk for developing an addiction, however, varies considerably between individuals, and different people can have very different responses to drug or stress exposure. One means for addressing this issue is examining animals with divergent behavioral phenotypes in appropriate neuronal structures. Akin to human addicts, inbred Lewis (L) rats self-administer opiates at high levels (George and Goldberg, 1989), and show escalation of drug-taking behavior (Picetti et al, 2012). Additionally, L rats display several anxiety-like phenotypes as compared with outbred Sprague-Dawley (SD) rats, as well as hypocortisolemia; thus, they have been suggested to be a good model of post-traumatic stress disorder (PTSD) (Cohen et al, 2006).
The bed nucleus of the stria terminalis (BNST) is a forebrain nucleus in the extended amygdala positioned to relay between cortical, hippocampal and amygdalar inputs, and stress and reward centers (Drolet, 2009). The BNST is densely innervated by noradrenergic fibers arising from the A1, A2 (via the ventral noradrenergic bundle or VNAB), and A6 (via the dorsal noradrenergic bundle) cell bodies (Forray and Gysling, 2004). NE signaling within the BNST modulates anxiety-like behavior and influences induction of the hypothalamic–pituitary–adrenal (HPA) axis (Cecchi et al, 2002), affects expression of learned and physical opiate withdrawal behaviors (Delfs et al, 2000) and contributes to stress-induced reinstatement of drug seeking (Erb et al, 2000; Leri et al, 2002; Wang et al, 2001). Furthermore, NE impacts both excitatory (McElligott and Winder, 2009) and inhibitory synaptic transmission (Dumont and Williams, 2004), induces synaptic plasticity (McElligott and Winder, 2008), and releases corticotropin-releasing factor (CRF) in the BNST (McElligott et al, 2010; Nobis et al, 2011). The Morilak lab found that extracellular NE in the BNST was the same in non-manipulated L and SD rats; however, when restrained for 30 min, extracellular NE was elevated in L rats compared with SD rats (Pardon et al, 2002). Furthermore, L rats exhibit fear generalization and anxiety-like behaviors that are BNST dependent (Duvarci et al, 2009).
Here, we hypothesized that the noradrenergic system projecting to the BNST may be differently regulated in these two rat strains. Furthermore, we tested whether the noradrenergic system is differentially modulated in a model of morphine addiction. Previously, we demonstrated that NE dynamics and their regulation can be examined in the BNST with fast-scan cyclic voltammetry (Herr et al, 2012; Park et al, 2009). We used this technique in the ventral BNST (vBNST) to investigate the mechanisms underlying differences in NE overflow in L rats as compared with SD rats, and to test the hypothesis that morphine dependence alters regulatory mechanisms at noradrenergic neurons. We found that non-manipulated L rats had a reduced rate of clearance and decreased sensitivity at their α2-adrenergic receptors (ARs) as compared with SD rats. Furthermore, using high-performance liquid chromatography (HPLC), we found that non-manipulated L rats had higher NE tissue content. When SD rats were made physically dependent on morphine (Schulteis et al, 1999), modeling the condition of a human addict, there was a significant reduction in their NE uptake rate and in their response to a challenge with an α2-AR antagonist as compared with controls. In contrast, neither clearance rate nor α2-AR sensitivity were different in morphine-dependent L rats as compared with control or non-manipulated animals. Correlated to these alterations at NE synapses in the BNST, morphine-dependent SD rats, but not L rats, showed heightened anxiety-like behavior as compared with controls. The changes that occurred in morphine-dependent SD rats were profound: they exhibited indistinguishable NE uptake rates and similar responses to an α2-AR antagonist as non-manipulated L rats. Thus, noradrenergic synapses underwent a remarkable adaptation when SD rats were made physically dependent on morphine. Furthermore, the data revealed that the vBNST NE system of the non-manipulated L rat resembled a ‘morphine-dependent state'.
MATERIALS AND METHODS
Animals Care
All experiments were performed in accordance with the University of North Carolina at Chapel Hill (UNC) Institutional Animal Care and Use Committee's guidelines. SD and L rats (males; 350–450 g; L rats total n=70, SD total n=85; Charles River, Wilmington, MA) were housed within UNC animal facilities and given food and water ad libitum. Electrochemical, biochemical, and behavioral experiments were performed as follows: voltammetry, SD n=34, L n=26; HPLC, SD n=22, L n=15; autoradiography, SD n=14, L n=13; elevated plus maze, SD n=15, L n=16.
Evoked NE Release
NE release in the vBNST was evoked from stimulation of the VNAB as previously described (Park et al, 2009) (Supplementary Methods).
Determination of NE and Dopamine Content in Tissue Slices
Brains were rapidly removed from anesthetized rats (urethane 1.5 mg/kg). Coronal sections (300 μm thick) were prepared using a Lancer Vibratome (World Precision Instruments, Sarasota, FL) in 4 °C artificial cerebral spinal fluid (aCSF). The aCSF (in mM: 126 NaCl, 25 NaHCO3, 2.45 KCl, 12 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 20 HEPES, and 11 glucose) was adjusted to pH 7.4 and oxygenated (95% O2/5% CO2). Tissue containing the vBNST or nucleus accumbens (NAc) was excised with a 1-mm punch, and homogenized in 0.1 N HClO4 containing 1 μM hydroquinone. Tissue processing and HPLC were performed as previously described (Park et al, 2009) (Supplementary Methods).
Acute Morphine Dependence
The morphine-dependence protocol was modified from Schulteis et al, 1999 (Supplementary Methods). Briefly, over the course of 3 days rats were injected once daily with 10 mg/kg morphine s.c., followed 4 h later by 1 mg/kg naloxone s.c. Behavioral, electrochemical, and autoradiography experiments were performed on the fourth day.
Elevated Plus Maze
The elevated plus maze (EPM) was used to assay anxiety-like behavior. Animals were isolated in a novel cage for 5 min before running the maze. Animals explored the maze for 5 min and their movement and time spent in each section (open arms, center, enclosed arms) was measured and recorded using Ethovision software (Noldus, Netherlands).
Chemicals and Drugs
All chemicals and drugs were purchased from Sigma-Aldrich (St Louis, MO), with the exception of naloxone (Tocris Bioscience, Ellisville, MO), and used as received. Drugs were dissolved in sterile saline (0.9%).
Statistics
Results are average values ± SEM. Mainly unpaired Student's t-tests and 2-way analysis of variance (ANOVA) with post-hoc Bonferroni tests were used to determine statistical significance. Morphine withdrawal scores, pre-withdrawal weight, and fecal boli weight were compared using a 3-way ANOVA with a post-hoc Tukey's Honestly Significant Difference (HSD) Test. Linear regression analysis was used to examine the relationship between stimulation intensity and [NE]max. Differences were considered significant when *P<0.05, **P<0.01, ***P<0.0001.
RESULTS
L Rats had Greater NE Tissue Content
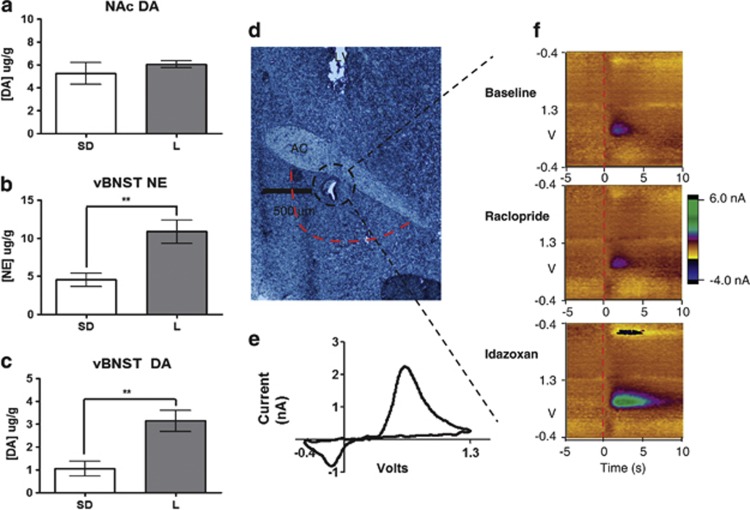
Owing to the phenotypes discussed above, we investigated the catecholamine tissue content in the nucleus accumbens (NAc) and the vBNST in L rats compared with SD rats. We did not see significant differences between the strains in the levels of DA in the NAc (SD: 5.25±0.96 μg/g n=5, L: 6.06±0.32 μg/g n=5); however, in the vBNST, L rats had significantly more DA and NE tissue content (SD NE: 4.55±0.84 μg/g n=5 vs L NE: 10.9±1.52 μg/g n=5; SD DA: 1.06±0.32 vs 3.15±0.46 n=5; Figure 1 a–c). We presumed the DA in the vBNST was mainly localized in NE neurons because DA is the metabolic precursor to NE, and dopaminergic projections are mainly to the dorsal lateral BNST (Park et al, 2012; Meloni et al, 2006).
Figure 1.
Fast-Scan Cyclic Voltammetry of norepinephrine (NE) in the bed nucleus of the stria terminalis (BNST). (a–c) Tissue content analysis of catecholamines in the BNST and nucleus accumbens (NAc). (d) Representative histology of recording electrode placement in the ventral BNST (vBNST) (black dashed circle) and area excised for tissue content analysis (red dashed line; LV, lateral ventricle; AC, anterior commissure). (e) Representative cyclic voltammogram of NE at [NE]max. (f) Representative color plots demonstrating NE release and uptake in baseline conditions, following 2 mg/kg raclopride, or 5 mg/kg idazoxan.
L Rats had Altered Noradrenergic Neurotransmission Compared with SD Rats
Next, we examined regulation of noradrenergic synaptic function using electrically-evoked release measured with fast-scan cyclic voltammetry in anesthetized rats. This method has been used extensively to characterize regulation of DA and serotonin neurotransmission (John et al, 2006), and we recently demonstrated its use to measure NE dynamics in vivo in the vBNST (Herr et al, 2012; Park et al, 2009). With the carbon-fiber microelectrode positioned directly beneath the anterior commissure, NE release was evoked by electrical stimulation of the VNAB (example electrode placement shown in Figure 1d). NE release (shown in color plots encoding voltammetric recordings over a 15-s interval) occurred upon stimulation, and its concentration fell to its original value after stimulation. Consistent with our previous report (Park et al, 2009), NE evoked release was unaffected by raclopride (2 mg/kg), a D2 receptor antagonist. In contrast, idazoxan (5 mg/kg, IDA), an α2–AR antagonist, increased NE evoked release (examples in Figure 1f).
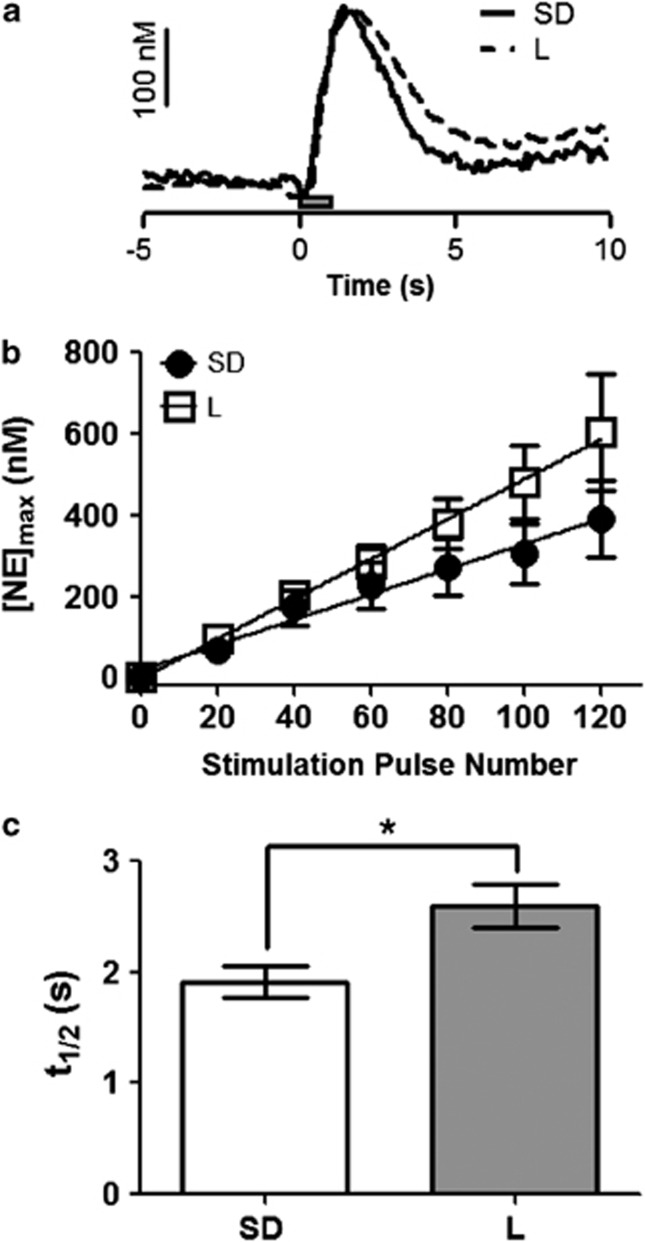
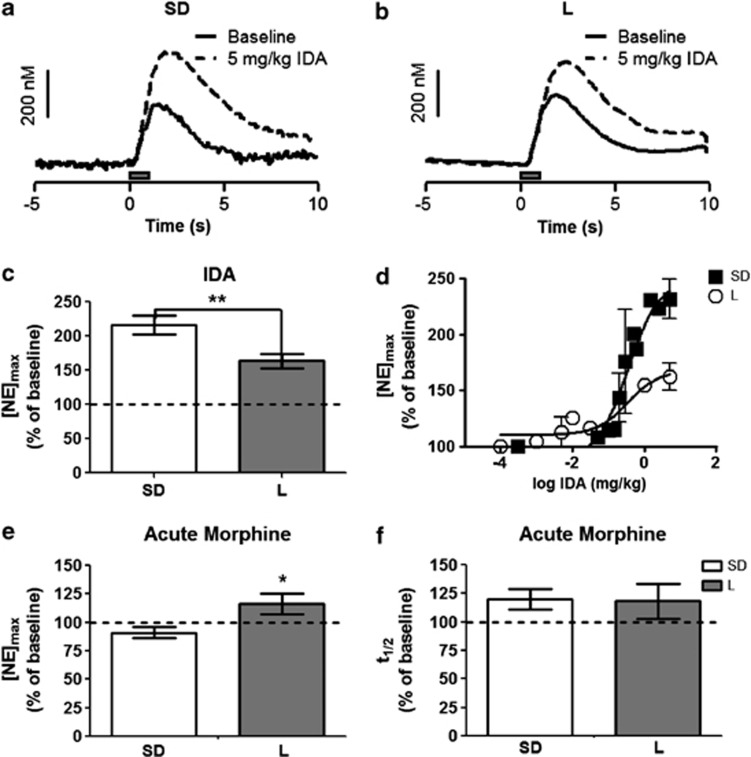
We compared stimulated release of NE in the vBNST in L and SD rats. The maximal amplitude of stimulated release ([NE]max) was evaluated as a function of pulse number in the stimulus train (at 60 Hz) and fit with a linear regression. Both strains responded to stimulation linearly, although the slope of the line was greater in the L rats (L vs SD, respectively, r2=0.98 vs 0.99, slope: 4.93±0.11 vs 3.11±0.21, P<0.05; Figure 2b). The clearance half-life (t1/2 or time from the [NE]max to half the maximal concentration) following 60 Hz 60 pulse stimulations was significantly greater in L rats than in SD rats (2.6±0.2 s, n=6 vs 1.9±0.1 s, n=11 respectively, P<0.05; Figure 2c). Additionally, when a saturating dose of the α2-AR antagonist IDA (5 mg/kg i.p.; see Figure 3 for dose response curve) was administered under the same stimulation conditions to L rats, the increase in [NE]max was significantly reduced compared with SD rats (163±11% of pre-drug baseline, n=8 vs 217±14% of pre-drug baseline n=10, respectively, P<0.05; Figure 3c), further suggesting that NE dynamics are different in these strains. Autoradiography for α2-AR and the norepinephrine transporter (NET), however, showed no differences between the strains (SD n=4, L n=4, Supplementary Figure 1),suggesting total protein levels are the same.
Figure 2.
Comparison of norepinephrine (NE) dynamics in naïve Lewis (L) and Sprague-Dawley (SD) rats. (a) Representative concentration traces demonstrating NE release and uptake in the ventral bed nucleus of the stria terminalis (vBNST) of both SD and L rats (scale bar, 100 nM; gray box =stimulation, 60 pulses, 60 Hz). (b) Input–output curve of [NE]max at 20, 40, 60, 80, 100 and 120 pulses in SD and L rats. (c) Histogram of mean clearance half-life of NE following 60 Hz, 60 pulse stimulation in both SD and L rats.
Figure 3.
Effect of idazoxan (IDA) and morphine on norepinephrine release in the ventral bed nucleus of the stria terminalis (vBNST). (a and b) Representative concentration traces of Sprague-Dawley (SD) and Lewis (L) rats, respectively. Solid line—evoked norepinephrine (NE) concentration with 60 Hz, 60 pulses stimulation; dashed line—evoked NE concentration following 5 mg/kg IDA with the same electrical stimulus. (c) Average [NE]max following 5 mg/kg IDA relative to pre-drug stimulated release. (d) Change in [NE]max as percent of pre-drug baseline in both Sprague-Dawley (closed squares) and Lewis (open circles) rats, plotted vs log concentration of IDA (n=3 each strain). (e) Average [NE]max to acute 5 mg/kg morphine relative to pre-drug stimulated release. (f) Average clearance half-life following acute 5 mg/kg morphine relative to pre-drug stimulated release.
Following this characterization, we probed the effect of an acute morphine injection (5 mg/kg i.p.) on NE dynamics. Surprisingly, the acute morphine slightly but significantly increased [NE]max in L rats but not in SD rats (116±9% of pre-drug baseline, n=5, P<0.05 vs 91±5% of pre-drug baseline, n=5; Figure 3e). There was no change in t1/2 in either strain following acute morphine (L rats: 118±15% of pre-drug baseline, n=5; SD rats: 120±12% of pre-drug baseline, n=9; Figure 3f). Furthermore, when the response to IDA was compared in acutely treated and non-manipulated animals, there was a main effect of strain (2-way ANOVA, F=9.41, P<0.01; SD rats: 191±12.8% vs 217±14% of pre-drug baseline, n=5 and 10,with and without morphine, respectively; L rats: 158.6±5.4% vs 163±11% of pre-drug baseline, n=5 and 8, with and without morphine, respectively ) but not of drug treatment. Therefore, acute morphine alone did not alter IDA response in either strain.
Morphine-Dependent SD Rats had Increased Anxiety-like Behavior
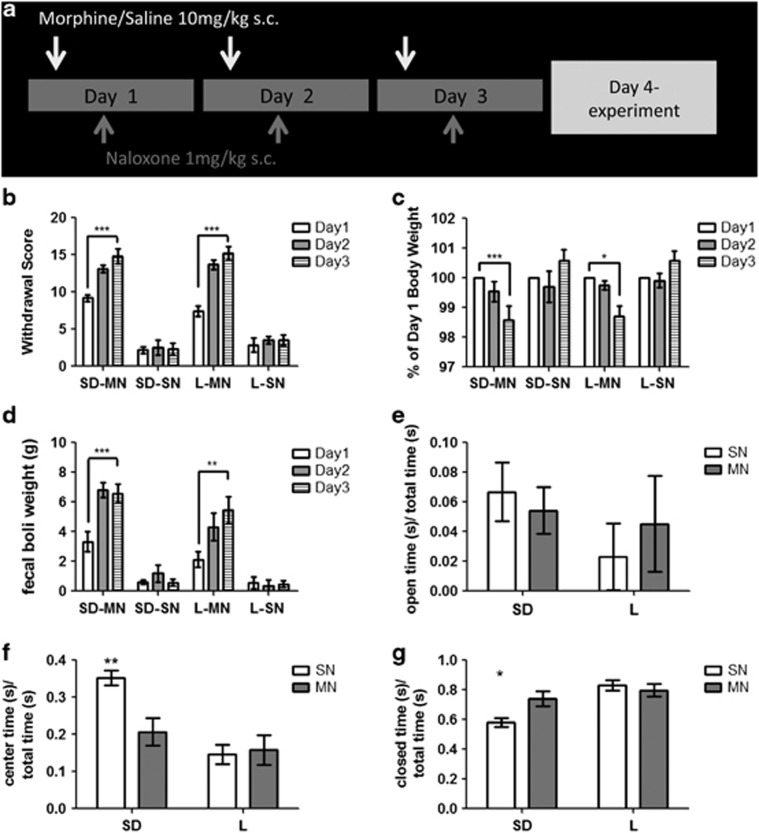
Next, we assayed animals that had experienced morphine withdrawal using a dependence paradigm that modeled the negative affect experienced by human addicts (Amitai et al, 2006; Koob, 2009; Schulteis et al, 1999). Once daily, we administered 10 mg/kg morphine s.c., followed by 1 mg/kg naloxone s.c. 4 h later (denoted MN), for a total of 3 days (SD n=27, L n=22). Control animals received a saline injection s.c. followed by 1 mg/kg naloxone 4 h later (denoted SN) once daily for a total of 3 days (SD n=26, L n=23). For 10 min following the injection of naloxone, animals were assayed for withdrawal behaviors (Supplementary Methods) and assigned a global withdrawal score (Figure 4a). There was a significant effect of drug and day on the global withdrawal score, but not of strain (3-way ANOVA, F=25.34, P<0.0001; Figure 4b). Thus, SD and L rats that received morphine had significantly greater withdrawal symptoms than their saline control counterparts. By the third day, both strains that received morphine showed withdrawal scores that were significantly different from the first day, while saline controls did not (Tukey's HSD Test, P<0.0001). Furthermore, there was an effect of day and treatment, but not strain, with regard to pre-withdrawal body weight (F=15.99, P<0.001). Both strains that received morphine had significant decreases in pre-withdrawal body weight (day 3 vs day 1, Tukey's HSD Test, P<0.01 SD, P<0.05 L; Figure 4c). As a quantitative measure of withdrawal-induced gut motility, we weighed the fecal boli produced during the 60 min following naloxone administration. Again, we found a significant effect of treatment but not strain (F=59.08, P<0.0001) and a significant increase in day 3 vs day 1 fecal boli weight (Tukey's HSD Test, P<0.001 SD, P<0.01 L; Figure 4d). The similarity in responses to the morphine/naloxone challenge shows that morphine dependence was induced in both strains. Owing to the short half-lives of morphine and naloxone (Trescot et al, 2008), both drugs were eliminated by experimentation time on day 4.
Figure 4.
Morphine dependence in Sprague-Dawley (SD) and Lewis (L) rats. (a) Model of drug administration paradigm. (b) Global withdrawal scores, (c) pre-withdrawal body weight (as % of day 1), and (d) fecal boli weight in SD morphine/naloxone (SD-MN) and L-MN rats following withdrawal, compared with control SD saline/naloxone (SD-SN) and L-SN rats. Time spent in (e) open arms (f), center or (g) closed arms of the elevated plus maze as a fraction of total time in the maze in SD-MN, SD-SN, L-MN, and L-SN rats.
We next examined MN and SN animals of both strains on the EPM. Both center and enclosed arm time showed an interaction (2-way ANOVA, center: F=5.78, P<0.05; enclosed: F=5.49, P<0.05) and a main effect of strain (2-way ANOVA, center: F=15.2, P<0.001; enclosed: F=14.0, P<0.001). Post-hoc analysis revealed significant differences between SD-MN and SD-SN time spent in the center (62±11 s vs 106±6 s, n=8 and 7, respectively, P<0.01) and the enclosed arms (222±15 s vs 175±9 s respectively, P<0.05). L-MN rats, however, failed to show any differences in time spent in the center or enclosed arms when compared with L-SN rats (center: 47±12 s vs 44±8 s, enclosed: 239±13 s vs 250±11 s, respectively, n=8; Figure 4 e–g). No significant differences were noted in number of entries or open arm time between groups.
NE Dynamics were Altered in Morphine-Dependent SD Rats but not L Rats
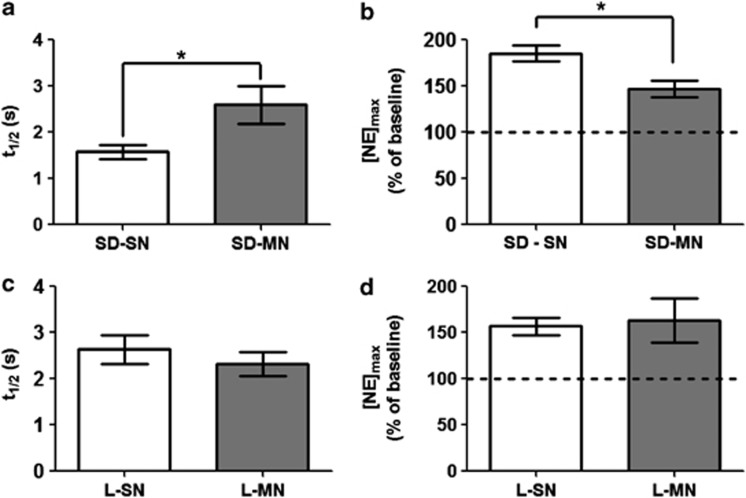
After establishing morphine dependence, animals were anesthetized on day 4 and NE overflow was measured in the vBNST using fast-scan cyclic voltammetry. We found that the t1/2 in SD-MN rats was significantly longer than in SD-SN rats (2.6±0.4 s vs 1.6±0.2 s, n=5 and 6, respectively, P<0.05; Figure 5a). Additionally the increase in [NE]max, following IDA was significantly blunted in the SD-MN rats as compared with SD-SN rats(147±9% vs 186±8%, n=5 and 6, respectively, P<0.05; Figure 5b). Interestingly, neither t1/2 nor the response to IDA was altered by morphine dependence in the L rats (t1/2: L-MN 2.3±0.3 s vs L-SN 2.6±0.3 s, IDA: L-MN 163±25% of pre-drug baseline vs L-SN 157±10% of pre-drug baseline, n=5; Figure 5c and d). Neither SD-MN, L-MN, nor L-SN groups were significantly different from the non-manipulated L group with respect to either t1/2 or response to the IDA challenge. Similar to non-manipulated animals, we did not observe changes in α2-AR or NET binding in the vBNST of either strain or condition (SD-MN n=5, SD-SN n=5, L-MN n=4, L-SN n=5, Supplementary Figure 1).
Figure 5.
Clearance half-life and change in response following idazoxan (IDA) administration in morphine/naloxone (MN) animals vs saline controls. (a) t1/2 and (b) change in [NE]max as percent of pre-drug baseline in MN Sprague-Dawley (SD) rats vs saline controls (P<0.05). (c) t1/2 and (d) change in [NE]max as percent of pre-drug baseline in MN Lewis (L) rats vs saline controls (n.s.). SN, saline/naloxone.
Catecholamine Tissue Content in Morphine-Dependent and Control Rats
Finally, we compared the catecholamine tissue content in the BNST and NAc of MN animals and their controls (Table 1). Contrary to naïve animals, when NE tissue content was examined there was no significant interaction between MN and SN animals of each strain (2-way ANOVA, SD-MN: 1.97±0.49 μg/g, n=9; SD-SN 3.23±0.72 μg/g, n=8; L-MN: 4.36±0.70 μg/g, n=5; L-SN: 3.12±0.65 μg/g, n=5 P>0.05). When BNST DA levels were examined, however, there was a main effect of both strain and drug treatment (2-way ANOVA, SD-MN: 1.32±0.22 μg/g, n=9; SD-SN 0.71±0.17 μg/g n=8; L-MN: 2.69±1.02 μg/g, n=5; L-SN: 1.55±0.59 μg/g, n=5, P<0.05 for each factor). Interestingly, when DA levels in the NAc were measured, there was a significant interaction (2-way ANOVA, SD-MN: 5.21±0.91 μg/g, n=9; SD-SN 4.85±0.75 μg/g n=8; L-MN: 7.25±1.31 μg/g, n=5; L-SN: 2.37+0.46 μg/g, n=5 P<0.05). Post-hoc analysis revealed that L-MN rats had significantly higher DA in the NAc than their L-SN controls (P<0.05), whereas SD animals showed no difference.
Table 1. Catecholamine Tissue Content Analysis (μ g/g) in the Nucleus Accumbens and Ventral Bed Nucleus of the Stria Terminalis of Control (Saline/Naloxone) and Morphine-Dependent (Morphine/Naloxone) Rats by HPLC.
| DA NAc (μg/g) | NE vBNST (μg/g) | DA vBNST (μg/g) | |
|---|---|---|---|
| Sprague-Dawley | |||
| Saline/naloxone (n=8) | 4.85±0.75 | 3.23±0.72 | 0.71±0.17 |
| Morphine/naloxone (n=9) | 5.21±0.91 | 1.97±0.49 | 1.32±0.22 |
| Lewis | |||
| Saline/naloxone (n=5) | 2.37±0.46* | 3.12±0.65 | 1.23±0.37 |
| Morphine/naloxone (n=5) | 7.25±1.31* | 4.36±0.70 | 2.69±1.02 |
*P<0.05.
DISCUSSION
Previous studies have shown that L rats have anxiety and PTSD-like phenotypes (Cohen et al, 2006) and elevated extracellular NE in the BNST in response to a stressor (Pardon et al, 2002). They are also predisposed to substance abuse (Picetti et al, 2012; Sanchez-Cardoso et al, 2007). Here, we found that the tissue content of NE and DA in the vBNST, but not DA in the NAc, was elevated in L rats as compared with SD rats. Furthermore, using fast-scan cyclic voltammetry, we uncovered profound differences in the regulation of NE synaptic function in the vBNST in the two strains. NE is less regulated in L rats when compared with SD rats because they have a reduced NE uptake rate and less sensitivity to an α2-AR antagonist. When SD rats were made dependent on morphine the two key regulators of NE overflow, uptake, and autoreceptor regulation, were both reduced such that NE neurotransmission resembled that of a non-manipulated L rat. In L rats, however, NE clearance and autoreceptor regulation were unaffected by morphine dependence. This physiological phenomenon correlated to an increase in anxiety-like behavior in SD-MN rats but not L-MN rats, as compared with their controls. The results demonstrate a robust plasticity within an organism's noradrenergic system that may be influenced by genetic factors as well as environmental insults.
L Rats Exhibited Poor Control Over NE Release and Clearance
Neurotransmitter levels in the extracellular space are determined by the balance between release and uptake (Wightman et al, 1988). Release is a function of the rate of impulse flow (action potentials), the relative amount of NE in the releasable pool, and regulation by autoreceptors. The principal inhibitory autoreceptor in noradrenergic neurons is the α2 -AR (Trendelenburg et al, 2001). NET is primarily responsible for NE clearance by uptake (Xu et al, 2000), and it follows Michaelis–Menten kinetics (Park et al, 2009). Electrical stimulation of NE neurons provides a convenient way to monitor release and uptake. Stimulation at supraphysiological frequencies allows release to predominate over uptake, enabling its nature to be examined. The maximum concentration achieved ([NE]max), measured at the end of the stimulation, is thus predominantly a function of release. Recently the Jones lab has shown that the t1/2 after the stimulation provides a measure of the rate of uptake that agrees with more complex Michaelis–Menten modeling (Yorgason et al, 2011). Thus, the electrical stimulations used here allowed quantification of several basic parameters that control extracellular NE.
Our studies showed that [NE]max is similar in the two strains with shorter stimulation trains. This is surprising because the vBNST NE content is twice as large in naïve L rats compared with SD rats. While release is linear in both strains, the slope of the regression line in the SD rats was significantly less than L rats, indicating that L rats have a larger releasable pool (Montague et al, 2004). Microdialysis studies have shown that extracellular NE increases during restraint stress in L rats relative to SD rats (Pardon et al, 2002), possibly arising from the larger releasable pool revealed in this work. Additionally, our experiments that probed the efficacy of the autoreceptor revealed that it exerts greater inhibition of release in SD rats. Although urethane anesthesia has been shown to stimulate α2-ARs in the periphery (Armstrong et al, 1982), our results in anesthetized animals mimicked the IDA effects observed in awake rats (Park et al, 2012). Taken together, our results suggest that L rats appear optimized for maximal NE neurotransmission. The combination of high content, low uptake rate, and low autoreceptor regulation ensures enhanced NE tone.
NE signaling in the BNST has been implicated in mediating control over the HPA axis and in anxiety-like behavior (Cecchi et al, 2002) and can release CRF within the BNST (McElligott et al, 2010; Nobis et al, 2011). Furthermore, prolonged NE signaling in the BNST induces synaptic plasticity that is modulated by stress (McElligott et al, 2010; McElligott and Winder, 2008). The altered mechanisms in L rats that allow enhanced NE signaling may result in their anxiety-like phenotypes observed by others (Cohen et al, 2006), and could translate to anxiety-susceptible human populations. Indeed, recent data show that targeting noradrenergic signaling mechanisms may alleviate symptoms of substance abuse and PTSD (Raskind et al, 2000; Simpson et al, 2008).
Morphine Dependence Enhanced Anxiety-Like Behavior, Increased NE Clearance Half-Life and Reduced Autoreceptor Function in SD but not L Rats
It is hypothesized in the allostasis model that the progression to addiction requires the engagement of neuronal stress/anxiety systems, including the release of NE in the BNST (Delfs et al, 2000; Koob, 2009). Therefore, a predisposition to the negative emotional state associated with engagement of these neuronal substrates might explain the co-morbidity that is associated with anxiety disorders and addiction. L rats have a high anxiety/PTSD phenotype that is at least partially due to BNST function (Cohen et al, 2006; Duvarci et al, 2009) and a propensity to self-administer and escalate opiate intake (Picetti et al, 2012; Sanchez-Cardoso et al, 2007). Interestingly, following withdrawal from morphine, but not acute morphine, SD rats showed profound plasticity in both autoreceptor regulation and uptake rates, as well as enhanced anxiety-like behavior. Moreover, the SD-MN rats had uptake rates and autoreceptor profiles indistinguishable from non-manipulated L rats. Surprisingly, L-MN rats exhibited neither this plasticity nor further increases in anxiety-like behavior, suggesting that autoreceptors and uptake rates in the L rats may be in a maximally depressed state. The correlation between vBNST NE and increased anxiety revealed in this work implicate plasticity at noradrenergic synapses as one mechanism for the development of anxiety and addiction phenotypes.
Interestingly, our autoradiography experiments demonstrated that neither naïve, MN, nor SN rats of either strain showed alterations in α2-AR or NET levels in the vBNST. This may be due to several reasons. First, this technique probes targets that are on the cell surface and in internal pools; therefore, it does not exclusively examine functional receptors and transporters. Second, it is possible that the functional changes we observed are not due to alterations in protein level, but due to a post-translational modification. Finally, the BNST has strong expression of nonspecific organic cation transporters, which also may have a role in the clearance of NE (Gasser et al, 2009) and could be manipulated by the gene–environment interactions we probed here. Nevertheless, the functional results clearly showed that the synaptic dynamics are dramatically different between SD and L rats, and that they were greatly altered in SD-MN rats.
Examining catecholamine tissue content in MN and control rats showed interesting changes in our treatment groups. Our data demonstrated that naloxone treatment lowered NE levels in the BNST in both strains regardless of pretreatment with morphine, and the effect was long lasting. These data are intriguing because naloxone and other μ-opioid antagonists are prescribed to humans for opiate and alcohol dependence (Heilig and Egli, 2006). Further, we found that L-MN rats had elevated DA in their NAc as compared with their controls, which may also contribute to their self-administration escalation phenotype (Picetti et al, 2012).
We have shown that NE synapses in a genetic model of anxiety and addiction (L rats) permit exacerbated noradrenergic signaling due to reduced uptake and autoreceptor function. Furthermore, we showed that vBNST NE synapses in a relatively non-anxious SD rat resemble L rat NE synapses when SD rats are made morphine dependent. These data suggest that noradrenergic synapses undergo a plasticity event where increased NE signaling (here stemming from precipitated withdrawal) results in a biophysical/biochemical change within the presynaptic cell to permit prolongation of future NE signaling on post synaptic cells. Furthermore, these alterations correlated to enhanced anxiety-like behavior in the SD rats. Additionally, the data support the allostasis model (Koob and Volkow, 2010) and the idea that genetic factors contributing to negative affect could in turn increase susceptibility to developing substance abuse issues.
Acknowledgments
We thank Dr Michael Saddoris for advice on statistical methods, Dr Ryan Vetrano and Dr Fulton Crews for behavioral advice and assistance, and Elyse Dankoski and Dr Thomas Kash for comments on an earlier version of the manuscript. This work was supported by an NIH grant (NS15841) to RMW and the NIMH Psychoactive Drug Screening Program Contract to BLR.
BLR has received research support from Dai-Nippon Sumitomo and has been a consultant to Pfizer Pharmaceuticals, SeaChange Pharmaceuticals, Finnegan, Henderson, Faraboug, Garnett and Henderson LLP and Dai-Nippon Sumitomo Pharmaceuticals. BLR also receives payment from the Journal of Clinical Investigation for his duties as Associate Editor of the JCI. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Amitai N, Liu J, Schulteis G. Discrete cues paired with naloxone-precipitated withdrawal from acute morphine dependence elicit conditioned withdrawal responses. Behav Pharmacol. 2006;17:213–222. doi: 10.1097/00008877-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, Lefevre-Borg F, Scatton B, Cavero I. Urethane inhibits cardiovascular responses mediated by the stimulation of alpha-2 adrenoceptors in the rat. J Pharmacol Exp Ther. 1982;223:524–535. [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Drolet G. Progress in Neuro-Psychopharmacology and Biological Psychiatry. Elsevier Inc: Amsterdam; 2009. [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Compar Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- George FR, Goldberg SR. Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci. 1989;10:78–83. doi: 10.1016/0165-6147(89)90083-7. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol. 2012;107:1731–1737. doi: 10.1152/jn.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Hong KI, Chaplin TM, Dabre Z, Comegys AD, Kimmerling A, et al. A stress-coping profile of opioid dependent individuals entering naltrexone treatment: a comparison with healthy controls. Psychol Addict Behav. 2009;23:613–619. doi: 10.1037/a0017324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Budygin EA, Mateo Y, Jones SR. Neurochemical characterization of the release and uptake of dopamine in ventral tegmental area and serotonin in substantia nigra of the mouse. J Neurochem. 2006;96:267–282. doi: 10.1111/j.1471-4159.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, et al. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci USA. 2010;107:2271–2276. doi: 10.1073/pnas.0905568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon JrWA. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, et al. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biological psychiatry. 2011;69:1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci. 2009;30:2121–2133. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry. 2012;71:327–334. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology. 2012;220:163–172. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61:129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, et al. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–948. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–242. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, et al. A pilot trial of the alpha-1 adrenergic antagonist, Prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2008;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Klebroff W, Hein L, Starke K. A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice. Naunyn-Schmiedeberg Arch Pharmacol. 2001;364:117–130. doi: 10.1007/s002100100423. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11 (2 Suppl:S133–S153. [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, et al. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.