Abstract
Aim
The present study tested whether the selective monoacylglycerol lipase (MAGL) inhibitor JZL184 would reduce allodynia and paw edema in the carrageenan test.
Main methods
The anti-edematous and anti-allodynic effects of JZL184 were compared to those of PF-3845, an inhibitor of fatty acid amide hydrolase (FAAH), and diclofenac, a non-selective cyclooxygenase inhibitor. Cannabinoid receptor involvement in the anti-edematous and anti-allodynic effects of JZL184 was evaluated by administration of the respective CB1 and CB2 receptor antagonists rimonabant and SR144528 as well as with CB1(−/−) and CB2(−/−) mice. JZL184 (1.6, 4, 16, or 40 mg/kg) was administered for six days to assess tolerance.
Key findings
JZL184 administered before or after carrageenan significantly attenuated carrageenan-induced paw edema and mechanical allodynia. Complementary genetic and pharmacological approaches revealed that the anti-allodynic effects of JZL184 required both CB1 and CB2 receptors, but only CB2 receptors mediated its anti-edematous actions. Importantly, both the anti-edematous and anti-allodynic effects underwent tolerance following repeated injections of high dose JZL184 (16 or 40 mg/kg), but repeated administration of low dose JZL184 (4 mg/kg) retained efficacy.
Significance
These results suggest that the MAGL inhibitor JZL184 reduces inflammatory nociception through the activation of both CB1 and CB2 receptors, with no evidence of tolerance following repeated administration of low doses.
Keywords: Carrageenan, Pain, Allodynia, Inflammation, 2-arachidonylglycerol (2-AG), Monoacylglycerol lipase (MAGL), Endogenous cannabinoid, Anandamide, Fatty acid amide hydrolase, CB1 receptor, CB2 receptor
Introduction
The endogenous cannabinoid (endocannabinoid) system consists of two G-protein-coupled cannabinoid (i.e., CB1 and CB2) receptors (Gerard et al., 1991; Matsuda et al., 1990), the lipid endogenous ligands N-arachidonoylethanolamine (anandamide; AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995), and endocannabinoid biosynthetic and catabolic enzymes (Ahn et al., 2008). Whereas 2-AG binds to both cannabinoid receptors with similar affinity (Mechoulam et al., 1995), AEA possesses approximately four-fold higher affinity at CB1 receptors than CB2 receptors (Showalter et al., 1996). AEA and 2-AG are produced and released on demand, and are then rapidly metabolized by their respective major degradative enzymes, fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996, 2001) and monoacylglycerol lipase (MAGL) (Blankman et al., 2007; Dinh, 2004). These components of the endocannabinoid system represent potential therapeutic targets to treat obesity, psychiatric disorders, neuroinflammatory diseases, cancer, pain, and inflammatory conditions (Pacher, 2006). Accordingly, a growing body of research has demonstrated that FAAH or MAGL inhibition reduces nociceptive behavior in laboratory animal models of pain.
The bulk of research examining the role of endocannabinoid catabolic enzymes in nociception has focused on FAAH (Booker et al., 2011; Chang et al., 2006; Clapper et al., 2010; Jayamanne et al., 2006; Kinsey et al., 2011; Naidu et al., 2008, 2009, 2010; Suplita et al., 2005) largely because of a greater availability of selective FAAH inhibitors than selective MAGL inhibitors. The development of JZL184, a piperidine carbamate that preferentially and irreversibly inhibits MAGL, provided the first pharmacological tool that when administered acutely increases 2-AG brain levels, without altering AEA brain levels (Long et al., 2009). Systemic administration of JZL184 reduces nociceptive responses in the tail withdrawal, formalin, and acetic acid stretching tests (Busquets-Garcia et al., 2011, Long et al., 2009), and chronic constriction injury (CCI) model of neuropathic pain in mice (Kinsey et al., 2009). Intraplantar injection of JZL184 produces antinociception in the formalin test (Guindon et al., 2011) and capsaicin model of nociception (Spradley et al., 2010).
Although these findings indicate that MAGL inhibition reduces nociceptive behavior in multiple preclinical pain models, the effects of JZL184 have yet to be evaluated in a prolonged model of inflammatory nociception. Thus, in the present study we tested whether JZL184 would attenuate paw edema and mechanical allodynia in the carrageenan model of inflammatory pain. For comparison, we tested the nonsteroidal anti-inflammatory diclofenac and the FAAH inhibitor PF-3845, which has been shown to possess anti-inflammatory and anti-allodynic effects in complete Freund's adjuvant (Ahn et al., 2009), LPS (Booker et al., 2011), and CCI (Kinsey et al., 2009, 2010) pain models. Because repeated JZL184 treatment or genetic deletion of MAGL results in CB1 receptor functional tolerance (Chanda et al., 2010; Schlosburg et al., 2010), we also tested the impact of repeated administration of low and high doses of JZL184 on both dependent measures. Finally, we tested whether systemic administration of JZL184 after intraplantar carrageenan injections reverses edema and allodynia to infer whether this compound possesses efficacy to treat nociceptive behavior and edema following an inflammatory insult.
Methods
Subjects
Male C57BL/6 Jmice (Jackson Laboratory, Bar Harbor, ME) as well as male and female CB1 (−/−) and CB2 (−/−) mice and their respective littermate controls, CB1 (+/+) and CB2 (+/+) mice from the Center Transgenic Colony at Virginia Commonwealth University served as subjects. CB1 (−/−) and CB2 (−/−) mice were backcrossed onto a C57BL/6 J background for 13 and 6 generations, respectively. The subjects weighed between 18 and 25 g, and were housed four-five mice per cage in a temperature (20–22 °C) and humidity controlled AAALAC-approved facility. Mice were given unlimited access to food and water in their home cages and were maintained on a 12/12 h light/dark cycle. The sample size for each treatment group was 6 to 10 mice/group and for knockout studies was 4 to 9 mice/group. All animal protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (Institute of Laboratory and Animal Resources, 1996). After testing was completed, all mice were humanely euthanized via CO2 asphyxia, followed by rapid cervical dislocation.
Drugs
JZL184 and PF-3845 were synthesized as described previously (Ahn et al., 2009; Long et al., 2009) by Organix, Inc. (Woburn, MA). JZL184, PF-3845, the CB1 receptor antagonist rimonabant (SR141716, National Institute on Drug Abuse), and the CB2 receptor antagonist SR144528 (National Institute on Drug Abuse) were dissolved in a vehicle consisting of a mixture of ethanol, alkamuls-620 (Rhone-Poulenc, Princeton, NJ), and saline in a ratio of 1:1:18. The nonselective cyclo-oxygenase (COX) inhibitor diclofenac (DIC; Tocris, Ellisville, MO) was dissolved in saline. Each drug was given via the i.p. route of administration in a volume of 10 μl/g body weight.
Carrageenan-induced paw edema
Edema was induced by giving an intraplantar injection of 0.3% carrageenan (Sigma, St Louis) in a 20 ul volume using a 30 gauge needle into the hind left paw. Paw thickness was measured with electronic digital calipers (Traceable Calipers, Friendswood, TX), prior to and 5 h following carrageenan administration, which corresponds to peak edema (Wise et al., 2008). This procedure has been used previously by our laboratory (Cravatt et al., 2004; Lichtman et al., 2004; Wise et al., 2008).
Mechanical allodynia
The mice were placed inside ventilated polycarbonate chambers on an elevated aluminum mesh table and allowed to acclimate to the apparatus for 60 min before testing. Mechanical allodynia was assessed with von Frey filaments (North Coast Medical, Morgan Hill, CA), using the “up-down” method (Chaplan et al., 1994) 5 h after carrageenan administration. The plantar surface of each hind paw was stimulated five times with each filament (0.16–6.0 g), at a frequency of approximately 2 Hz, starting with the 0.6-g filament and increasing until the mouse responded by licking and/or lifting the paw off the surface of the test apparatus. Three or more responses out of five stimulations were coded as a positive response. Once a positive response was detected, sequentially lower weight filaments were used to assess the sensory threshold for each paw.
Testing procedures
Mice were transported to the testing room, weighed, randomly assigned to the different treatment regimens, and allowed to acclimate for at least 1 h before injections. The time course for carrageenan-induced paw edema was assessed in an initial experiment. Mechanical allodynia was assessed at the 5 h time point. For consistency with our previous studies, the 5 h time point was selected to assess paw edema and mechanical allodynia. The pre-treatment times for each drug were as follows: 30 min for diclofenac (5 mg/kg), 2 h for PF-3845 (1, 3, or 10 mg/kg), and 2 h for JZL184 (1.6, 4, 16, or 40 mg/kg). In experiments assessing cannabinoid receptor mechanism of action, the CB1 receptor antagonist rimonabant (1 mg/kg) and theCB2 receptor antagonist SR144528 (3 mg/kg) were administered30 min prior to JZL184 (16 mg/kg) or vehicle. It should be noted that in initial experiments, 3 mg/kg rimonabant reduced paw edema (data not shown). In contrast, 1 mg/kg rimonabant administered alone did not affect the dependent measures and was employed for the antagonism studies. Previous studies have shown that these doses of rimonabant (Lichtman et al., 1996; Lichtman and Martin, 1997, Lichtman et al., 2004) and SR144528 (Conti et al., 2002; Lichtman et al., 2004; Malan et al., 2002) block the pharmacological effects of cannabinoid receptor agonists. The anti-edematous and anti-allodynic effects of 16 mg/kg JZL184 were evaluated in CB1 (+/+), CB1 (−/−), CB2 (+/+), and CB2 (−/−) mice to further assess receptor involvement. In addition, we determined whether administration of the antagonists after carrageenan would reverse the anti-edematous and anti-allodynic effects JZL184. In this experiment, rimonabant (1 mg/kg) or SR144528 (3 mg/kg) was injected 4 h after carrageenan and edema and allodynia were measured at 5 h.
In order to assess the impact of repeated administration of JZL184 on paw edema and mechanical allodynia, the following groups of mice were tested: (Group 1) vehicle for 6 days, (Groups 2–5) vehicle for 5 days and challenged with 1.6, 4, 16 or 40 mg/kgJZL184onday 6, and (Groups 6–9) 1.6, 4, 16 or 40 mg/kg JZL184 for 6 days. Mice were administered their respective treatments 2 h before carrageenan was injected. Edema and mechanical allodynia were then assessed 5 h later. In the final experiment, JZL184 (16 mg/kg) was administered 3 h after carrageenan to examine whether carrageenan-induced edema and allodynia would be reversed at 5 h.
Data analysis
Paw edema data are expressed as the difference in paw thickness between the 5 h and pre-injection measures. Paw withdrawal thresholds to the von Frey filaments in the carrageenan-injected and contralateral (i.e., control) paws at the 5 h time point were used to assess mechanical allodynia. All data are depicted as mean±standard error of the mean (SEM). Data were analyzed using t-tests, one-way analysis of variance (ANOVA), or two-way ANOVA. Dunnett's test was used for post hoc analysis in the dose–response experiments in which the effects of each drug dose were compared to those of vehicle. The Tukey test was used for post hoc analysis to compare different treatment groups and Bonferroni planned comparisons were used to assess genotype differences. Differences were considered significant at the p<0.05 level.
Results
Anti-edematous and anti-allodynic effects of JZL184 in the carrageenan model
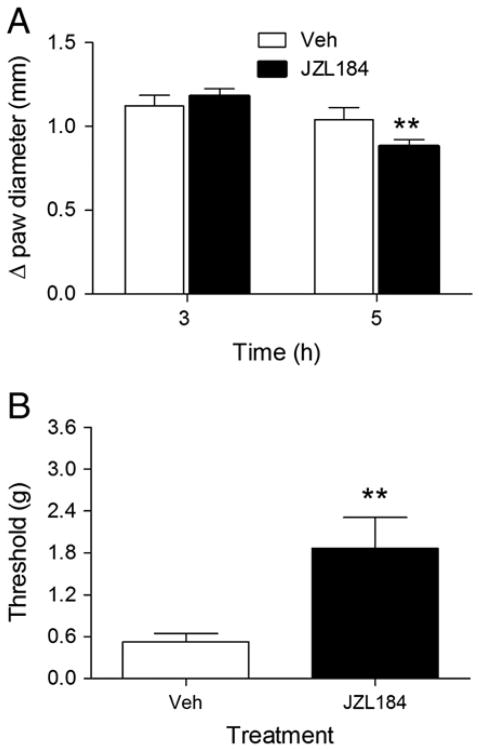
Intraplantar administration of carrageenan induced paw edema and decreased paw withdrawal threshold over an extended time period (Supplementary Fig. 1). In contrast, the withdrawal threshold for the control paw (i.e., contralateral paw) remained constant throughout all of the studies (Table 1). As shown in Fig. 1A, JZL184 [F (4, 33) = 24.64, p<0.001], PF-3845 [F (3, 20) = 25.76, p<0.001], and diclofenac [t (12) = 5.51, p<0.001] significantly attenuated carrageenan-evoked edema. Similarly, allodynia was significantly attenuated by JZL184 [F (4, 37) = 11.95, p<0.001], PF-3845 [F (3,20) = 6.596, p<0.05] and diclofenac [t (12) = 6.03, p<0.001]. None of the treatments altered the paw withdrawal threshold in the contralateral paw (Table 1).
Table 1.
The paw withdrawal threshold for the control paw was not altered by any of the treatments as measured with von Frey filaments. Values represent the mean (±SEM) mechanical paw withdrawal threshold.
| Experiment | Mean (g) | ±SEM |
|---|---|---|
| JZL184 acute dose | 3.27 | 0.142 |
| JZL 184 repeated dosing | 3.11 | 0.119 |
| PF-3845 acute dose | 3.20 | 0.276 |
| Diclofenac | 3.14 | 0.40 |
| Rimonabant | 3.35 | 0.165 |
| SR144528 | 2.90 | 0.248 |
| CB1 (−/−) mice | 3.37 | 0.297 |
| CB2 (−/−) mice | 2.96 | 0.217 |
| Rimonabant or SR144528 after carrageenan | 3.44 | 0.189 |
| JZL184 after carrageenan | 3.50 | 0.435 |
| JZL184 repeated administration | 3.21 | 0.164 |
Fig. 1.
JZL184, PF-3845, and diclofenac partially reduced edema and allodynia in the carrageenan model. JZL184 significantly reduced carrageenan-induced paw edema (Panel A) and allodynia (Panel B). Diclofenac and PF-3845 significantly attenuated carrageenan-induced paw edema (Panel C) and allodynia (Panel D). Values represent the mean (± SEM) mechanical paw withdrawal threshold and difference in paw thickness. *p<0.05, ***p<0.001 versus VEH. +++p<0.001 versus VEH. N = 6–7 mice/group.
Cannabinoid receptors mediate the anti-allodynic and anti-edematous of JZL184
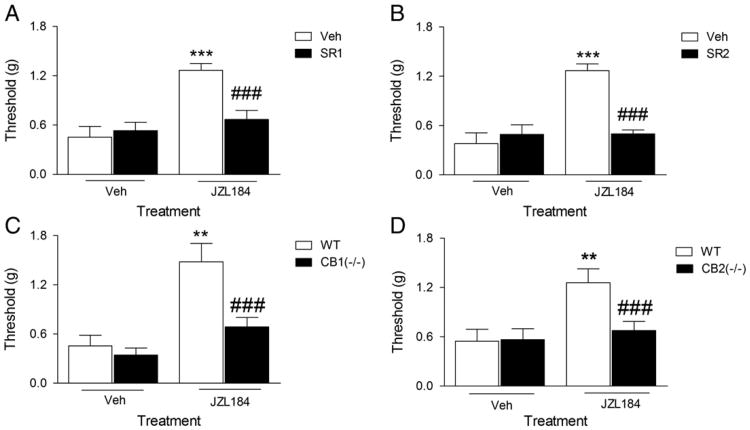
To determine whether CB1 and CB2 receptors mediate the anti-allodynic effects of JZL184, mice were pretreated with rimonabant (1 mg/kg), SR144528 (3 mg/kg), or vehicle 30 min prior to JZL184 (16 mg/kg) injection. As shown in Fig. 2A, rimonabant [F (1, 20) = 10.07, p<0.001] and SR144528 [F (1, 20) = 19.21, p<0.001] completely blocked the anti-allodynic effects of JZL184 (Fig. 2B). There was a significant interaction between JZL184 and pre-treatment with the rimonabant and SR144528 on mechanical allodynia, indicating the involvement of CB1 and CB2 receptors. Neither rimonabant nor SR144528 significantly affected allodynia when given alone. CB1 (−/−) [F (1, 19) = 5.73, p<0.001; Fig. 2C] and CB2 (−/−) [F (1, 24) = 8.74, p<0.001; Fig. 2D] mice were resistant to the anti-allodynic effects of JZL184. In the absence of drugs, both CB1 (−/−) and CB2 (−/−) showed similar nociceptive behavior as the wild type controls.
Fig. 2.
The anti-allodynic effects of JZL184 require both CB1 and CB2 receptors. Panel A. Rimonabant (SR1) blocked the anti-allodynic effects of JZL184. ***p<0.001 versus VEH/VEH; ###p<0.001 versus VEH/JZL184. Panel B. Similarly, SR144528 (SR2) blocked the anti-allodynic effects of JZL184. ***p<0.001 versus VEH/VEH; ### p<0.001 versus VEH/JZL184. Panel C. The anti-allodynic effects of JZL184 do not occur in CB1 (−/−) mice. **p<0.05 versus (+/+)/VEH, ### p<0.001 versus CB1 (+/+)/JZL184. Panel D. The anti-allodynic effects of JZL184 do not occur in CB2 (−/−) mice. **p<0.001 versus (+/+)/VEH, ### p<0.001 versus CB2 (+/+)/JZL184. Values represent the mean (± SEM) mechanical paw withdrawal threshold. N = 6/group.
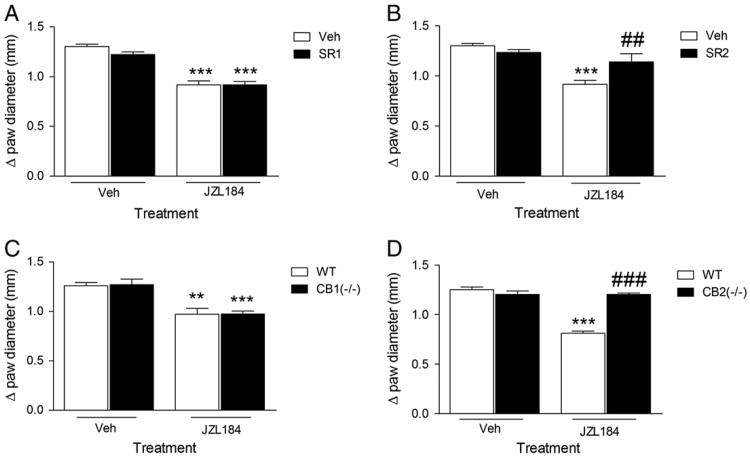
The data depicted in Fig. 3, reveal that CB2 receptors mediate the anti-edematous effects of JZL184. Whereas rimonabant was without effect [p = 0.22; Fig. 3A], SR144528, completely blocked the anti-edematous effects of JZL184, as revealed by a significant interaction between JZL184 and SR145528 [F (1, 20) = 8.73, p<0.001; Fig. 3B]. Consistent with the cannabinoid receptor antagonist data, JZL184 retained its anti-edematous effects in CB1 (−/−) mice [p = 0.84; Fig. 3C], but CB2 (−/−) mice were completely resistant to the anti-edematous effects of JZL184, as indicated by a significant interaction between JZL184 and the genotype [F (1, 21) = 59.97, p<0.001; Fig. 3D].
Fig. 3.
The anti-edematous effects of JZL184 require CB2 receptors, but CB1 receptors are expendable. Panel A. Rimonabant (SR1) did not block the anti-edematous effects of JZL184. ***p<0.001 versus VEH/VEH. Panel B. SR144528 (SR2) blocked the anti-edematous effects of JZL184. ***p<0.001 versus VEH/VEH; ## p<0.05 versus VEH/JZL184. Panel C. The anti-edematous effects of JZL184 occur in CB1 (−/−) mice. **p<0.01, ***p<0.001 versus (+/+)/VEH. Panel D. The anti-edematous effects of JZL184 do not occur in CB2 (−/−) mice (Panel D). ***p<0.001 versus (+/+)/VEH; ### p<0.001 versus (+/+)/JZL184. Values represent the mean (± SEM) difference in paw thickness. N = 5–7/group.
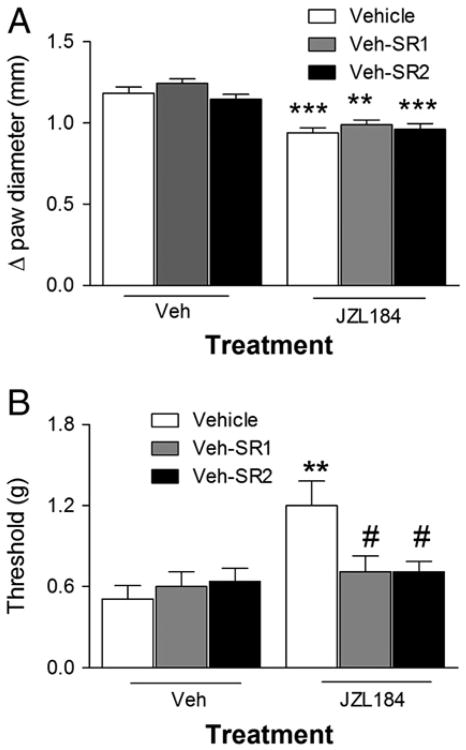
In the next series of experiments, we examined whether rimonabant or SR144528 injected 4 h after carrageenan would reverse the anti-edematous and anti-allodynic effects of JZL184 (16 mg/kg). The anti-edematous effects of JZL184 were not reversed by either drug [F (5, 27) = 15.53; Fig. 4A], but rimonabant as well as SR144528 completely reversed the anti-allodynic effects of JZL184 [F (5, 37) = 4.05, p<0.05; Fig. 4B].
Fig. 4.
JZL184-induced anti-allodynia occurs independently of its anti-edematous effect. Panel A. Neither rimonabant (SR1) nor SR144528 (SR2) blocked the anti-edematous effect of JZL184 when injected 4 h after carrageenan. Panel B. Both rimonabant and SR144528 blocked the anti-allodynic effect of JZL184 when injected 4 h after carrageenan. Values represent the mean (±SEM) mechanical paw withdrawal threshold and difference in paw thickness. **p<0.01, ***p<0.001 versus VEH/VEH; #p<0.05 versus JZL184/VEH. N = 6–9/group.
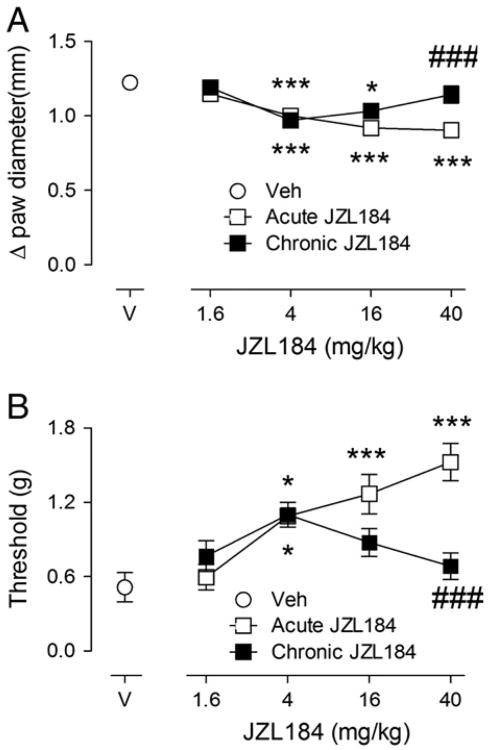
Differential tolerance following repeated administration of low dose and high dose JZL184
As genetic deletion or prolonged pharmacological inhibition of MAGL is known to produce CB1 receptor functional tolerance (Chanda et al., 2010; Schlosburg et al, 2010), we assessed the dose–response relationship of JZL184 (1.6, 4, 16, or 40 mg/kg) after acute or repeated administration in the carrageenan assay. JZL184 dose-dependently attenuated carrageenan-induced paw edema and allodynia. As shown in Fig. 5, acute administration of 4, 16, and 40 mg/kg JZL184 significantly attenuated carrageenan-induced edema [F (8, 65) = 11.62, p<0.001; Fig. 5A]. While the anti-edematous effects of 4 and 16 mg/kg JZL184 were maintained following repeated dosing, the anti-edematous effects of 40 mg/kg JZL184 underwent tolerance upon repeated administration. Acute administration of 4, 16, and 40 mg/kg JZL184 also significantly attenuated mechanical allodynia [F (8, 65) = 7.953, p<0.001; Fig. 5B]. The anti-allodynic effects of 4 mg/kg JZL184 was maintained after repeated dosing; however, repeated administration of high doses of JZL184 (16 and 40 mg/kg) led to tolerance. The lowest dose of JZL184, 1.6 mg/kg, tested in this study, remained ineffective regardless of whether it was administered acutely or repeatedly. There was no edema or allodynia seen in the contralateral paw (Table 1).
Fig. 5.
The anti-edematous (Panel A) and anti-allodynic (Panel B) effects of low doses of JZL184 do not undergo tolerance following repeated administration. *p<0.05, **p<0.01, ***p<0.001 versus VEH/VEH; ###p<0.001 versus acute 40 mg/kg JZL184. Values represent the mean (±SEM) mechanical paw withdrawal threshold and difference in paw thickness. N = 6–8/group.
JZL184 reverses carrageenan-induced anti-edema and allodynia
In the previous experiments, JZL184 administered before carrageenan decreased the development of paw edema and mechanical allodynia. In order to test whether JZL184 would reverse these responses, subjects received vehicle or JZL184 (16 mg/kg) 3 h after carrageenan. Edema was measured just before drug administration and at 5 h and allodynia was assessed at 5 h. As shown in Fig. 6A, JZL184 produced a significant partial reversal of carrageenan-induced edema at 5 h compared with the 3 h time point [F (1, 10) = 58.58, p<0.001]. JZL184 given after the induction of carrageenan-induced paw edema also significantly attenuated the mechanical allodynia at 5 h [Fig. 6B; t (10) = 2.90, p<0.01]. JZL184 did not affect paw withdrawal thresholds in control paws (Table 1).
Fig. 6.
JZL184 reverses edema (Panel A) and allodynia (Panel B) when administered 3 h after carrageenan. Drug was administered immediately after the 3 h allodynia assessment. Values represent the mean (± SEM) mechanical paw withdrawal threshold and difference in paw thickness. **p<0.01 versus JZL184 at 3 h. **p<0.01, versus VEH; N = 6/group.
Discussion
JZL184 represents the first selective MAGL inhibitor that elevates brain 2-AG, but not anandamide, levels following acute administration (Long et al., 2009). This compound reduces nociceptive behavior in a wide range of laboratory animal models of pain, including warm water tail withdrawal, acetic acid stretching, formalin, mechanical and cold allodynia following chronic constrictive injury of the sciatic nerve, capsaicin-induced mechanical allodynia, and bone cancer tests (Busquets-Garcia et al., 2011; Guindon et al., 2011; Khasabova et al., 2011; Kinsey et al., 2009, 2010; Spradley et al., 2010). The present study increases the understanding that MAGL inhibition plays on nociception by demonstrating that JZL184 reduces carrageenan-induced paw edema and associated mechanical allodynia. These effects were similar in magnitude to those produced by the FAAH inhibitor PF-3845, as well as the nonselective COX inhibitor diclofenac. The anti-edematous effects of JZL184 were mediated through CB2 receptors, while the anti-allodynic effects required both CB1 receptors and CB2 receptors. These findings are consistent with the observation that 2-AG has similar affinity for both cannabinoid receptors (Mechoulam et al., 1995). In addition, repeated administration of high dose JZL184 resulted in tolerance to its anti-allodynic and anti-edematous effects, but a low dose of JZL184 retained efficacy after repeated administration.
Initial studies investigating the in vivo consequences of inhibiting MAGL employed URB602 (Comelli et al., 2007; Desroches et al., 2008; Guindon et al., 2007a, 2007b, 2011; Guindon and Hohmann, 2008; Hohmann et al., 2005; Vandevoorde et al., 2007) and N-arachidonyl maleimide (Burston et al., 2008). For example, systemic or peripheral administration of URB602 suppressed formalin-induced nociception, carrageenan-induced inflammatory nociception, and partial sciatic nerve ligation-induced nociception in rats (Comelli et al., 2007, Desroches et al., 2008, Guindon et al., 2011) Although URB602 and N-arachidonyl maleimide inhibit MAGL in the brain, these compound are nonselective and inhibit other serine hydrolases, including FAAH (Burston et al., 2008; Hohmann et al., 2005; Vandevoorde et al., 2007).
A key finding in the present study was that JZL184 administration 3 h after carrageenan significantly reversed the magnitude of paw edema and mechanical allodynia. Similarly, URB602 reversed carrageenan-induced paw edema and paw withdrawal latency when administered after carrageenan in mice (Comelli et al., 2007). These findings suggest that endocannabinoid catabolic inhibitors may possess therapeutic utility in treating clinical symptoms associated with inflammatory disease states.
The observations that JZL184 did not attenuate carrageenan-induced edema in SR144528-treated wild type mice and CB2 (−/−) mice indicate a necessary role of CB2 receptors. In contrast, complementary pharmacological and genetic approaches indicated that CB1 receptors are expendable for the anti-edematous effects of JZL184. Likewise, the anti-edematous effect of URB602, which inhibits FAAH and MAGL with similar potency, has also been reported to be blocked by SR144528, but not by rimonabant (Comelli et al., 2007). Studies have shown that systemic and local administration of the CB2 receptor agonist AM1241 suppressed the development of mechanical stimulation in the carrageenan model of inflammation (Nackley et al., 2003a). This suppression was blocked by SR144528 but not by rimonabant (Nackley et al., 2003b). Previous studies have also reported that elevation of 2-AG attenuates inflammatory and immune response in vitro (Ouyang et al., 1998; Gallily et al., 2000; Facchinetti et al., 2003). Thus, inhibiting 2-AG degradation in vivo offers a strategy to augment levels of this endogenous cannabinoid to elicit anti-inflammatory effects through the stimulation of CB2 receptors.
Whereas the anti-edematous effect of JZL184 was mediated by CB2 receptors, its anti-allodynic effects required both CB1 and CB2 receptors. Similarly, the suppressive effects of JZL184 on capsaicin-induced thermal hyperalgesia and nocifensive behavior required both CB1 and CB2 receptors (Spradley et al., 2010). Likewise, intra-paw administration of JZL184 or URB602 produced antinociceptive effects in the formalin model, which were blocked by CB1 and CB2 receptor antagonists (Guindon et al., 2007a, 2011). In contrast, CB2 receptors did not play a necessary role in the anti-allodynic effects of JZL184 in the chronic constriction injury model (Kinsey et al., 2009, 2010). Two related questions are raised by these observations. First, why do both CB1 and CB2 receptors play a necessarily role in the antinociceptive effects of JZL184 in some types of nociceptive assays (e.g., carrageenan, formalin, and capsaicin), but only CB1 receptors are required in other models (e.g., warm water tail withdrawal, acetic acid stretching, CCI)? Second, what is the mechanism by which both cannabinoid receptors play a necessarily role in the anti-allodynic effects of JZL184 in the carrageenan assay? It is unlikely that the method to assess nociception accounts for these disparate findings because von Frey filaments were used to assess mechanical allodynia in both CCI and carrageenan assays. Instead, the differential cannabinoid receptor involvement may be related to the degree to which inflammatory responses contribute to the nociception as well as the concentration of the cannabinoid receptor subtypes and 2-AG at the critical sites of action. Indeed, JZL184 may be producing its actions at the site of inflammation in the paw, within the dorsal root ganglia or dorsal horn of the spinal cord, or at multiple supraspinal regions (e.g., PAG or the rostral ventromedulla). It has been shown that CB2 receptors are upregulated in the dorsal root ganglia and paw tissue of rodents administered complete Freund's adjuvant (Hsieh et al., 2011), suggesting that CB2 receptors in these regions could contribute to the findings reported here. For example, intraplantar carrageenan could lead to the infiltration of immune cells, such as macro-phages or neutrophils, at the site of injection, that express CB2 receptors (Galiegue et al., 1995). 2-AG activation of CB2 receptors on infiltrating cells might synergize with the well described antinociceptive actions of CB1 receptor stimulation on peripheral nociceptors (Agarwal et al., 2007) within the spinal cord (Yaksh, 1981; Lichtman and Martin, 1991) and within supraspinal sites of action (Lichtman et al., 1996; Martin et al., 1999). Future studies are needed to examine whether JZL184 alters inflammatory mediators (e.g, pro- and anti-inflammatory cytokines and prostaglandins, as well as infiltrating immune cells) caused by carrageenan. In order, to ascertain the relative contribution of CB1 and CB2 receptors in these effects, we also evaluated the ability of rimonabant and SR144528 administered 4 h after carrageenan to reverse the anti-allodynic and anti-edematous effects of JZL184. Whereas neither antagonist reversed the anti-edematous effects of JZL184, both rimonabant and SR144528 reversed the anti-allodynic effects of JZL184, indicating that these effects can be dissociated.
Another important finding in the present study was that the anti-allodynic and anti-edematous effects of JZL184 were maintained following repeated low dose administration, but the effects underwent tolerance after repeated high dose JZL184. Similarly, prolonged inactivation of MAGL via administration of high dose JZL184 (40 mg/kg) results in the loss of analgesic responses in the CCI model, cross-tolerance to exogenous cannabinoid receptor agonists (i.e., THC, and WIN55, 212-2), CB1 receptor downregulation and desensitization in cingulate cortex, hippocampus, somatosensory cortex, and PAG (Chanda et al., 2010; Schlosburg et al., 2010). Additionally, 2-AG elevation in MAGL (−/−) mice caused tonic activation and partial desensitization of CB1 receptors (Chanda et al., 2010; Schlosburg et al., 2010; Pan et al., 2011). The findings that the anti-edematous and anti-allodynic effects of low dose JZL184 were maintained after repeated dosing are consistent with a previous report in which repeated administration of 8 mg/kg JZL184 maintained its anxiolytic-like effects under high illumination conditions in the rat elevated plus maze assay (Sciolino et al., 2011).
Conclusion
In conclusion, the present study demonstrates that the selective MAGL inhibitor, JZL184, significantly inhibits inflammatory pain, as assessed in the carrageenan assay. More specifically, JZL184 attenuated the development of paw edema and mechanical allodynia and also reversed edema and allodynia when administered after carrageenan. Complementary genetic and pharmacological approaches revealed that the anti-allodynic effects of JZL184 required both CB1 and CB2 receptors, whereas only CB2 receptors had a necessary role in mediating its anti-edematous effect. We also found that the anti-allodynic and anti-edematous effects of low, but not high, doses of JZL184 do not undergo tolerance when administered repeatedly. These results indicate that the activation of CB1 and/or CB2 receptors by low doses of MAGL inhibitors may have beneficial effects on inflammatory pain that include the ability to prevent inflammation and reverse established inflammatory pain states.
Supplementary Material
Acknowledgments
We thank Dr. Divya Ramesh and Jason M. Wiebelhaus for their useful feedback throughout the project. This research was supported by the following grants from the National Institute on Drug Abuse: P01DA017259, P01DA009789, P50DA005274, R01DA03672, and R01DA005488.
Footnotes
Conflict of interest statement: No competing interests.
Appendix A. Supplementary data: Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.lfs.2012.06.020.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–20. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, et al. The FAAH inhibitor PF-3845 acts in the nervous system to reverse lipopolysaccharide-induced tactile allodynia in mice. Br J Pharmacol. 2011;165:2485–96. doi: 10.1111/j.1476-5381.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, et al. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–53. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–13. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–70. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br J Pharmacol. 2007;152:787–94. doi: 10.1038/sj.bjp.0707425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol. 2002;135:181–7. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–6. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–6. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches J, Guindon J, Lambert C, Beaulieu P. Modulation of the anti-nociceptive effects of 2-arachidonoyl glycerol by peripherally administered FAAH and MGL inhibitors in a neuropathic pain model. Br J Pharmacol. 2008;155:913–24. doi: 10.1038/bjp.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinh TP. RNA Interference Suggests a Primary Role for Monoacylglycerol Lipase in the Degradation of the Endocannabinoid 2-Arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–4. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41:161–8. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gallily R, Breuer A, Mechoulam R. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice. Eur J Pharmacol. 2000;406:R5–7. doi: 10.1016/s0014-2999(00)00653-1. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–34. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007a;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Dani M, Beaulieu P. Pre-emptive antinociceptive effects of a synthetic cannabinoid in a model of neuropathic pain. Eur J Pharmacol. 2007b;568:173–6. doi: 10.1016/j.ejphar.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Guindon J, Guijarro A, Piomelli D, Hohmann AG. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol. 2011;163:1464–78. doi: 10.1111/j.1476-5381.2010.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–34. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, et al. Central and peripheral sites of action for CB receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–40. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–8. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res. 2011;64:60–7. doi: 10.1016/j.phrs.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain. 2010;11:1420–8. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–10. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Naidu PS, Cravatt BF, Dudley DT, Lichtman AH. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav. 2011;99:718–25. doi: 10.1016/j.pbb.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258:517–23. [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–93. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol Biochem Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–27. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F. Inhibition of pain responses by activation of CB (2) cannabinoid receptors. Chem Phys Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res. 1999;822:237–42. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB (2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003a;119:747–57. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Suplita RL, II, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003b;117:659–70. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between Enzyme Inhibitors of Fatty Acid Amide Hydrolase and Cyclooxygenase in Visceral Nociception. J Pharmacol Exp Ther. 2008;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–90. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonylglycerol is mediated through down regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–83. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- Pacher P. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in mono-acylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–30. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–9. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–34. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–99. [PubMed] [Google Scholar]
- Spradley JM, Guindon J, Hohmann AG. Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms. Pharmacol Res. 2010;62:249–58. doi: 10.1016/j.phrs.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Suplita RL, 2nd, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49:1201–9. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–91. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54:181–8. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. The antinociceptive effects of intrathecally administered levonantradol and desacetyllevonantradol in the rat. J Clin Pharmacol. 1981;21:334S–40S. doi: 10.1002/j.1552-4604.1981.tb02612.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.