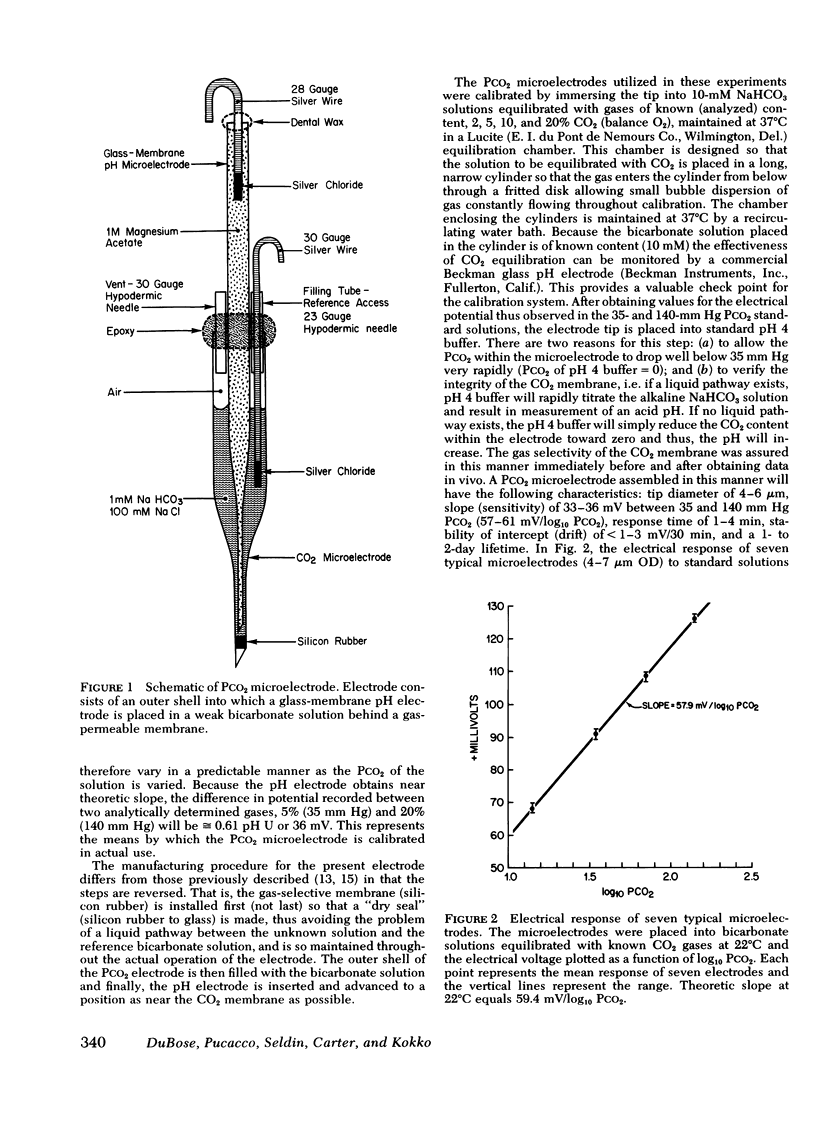
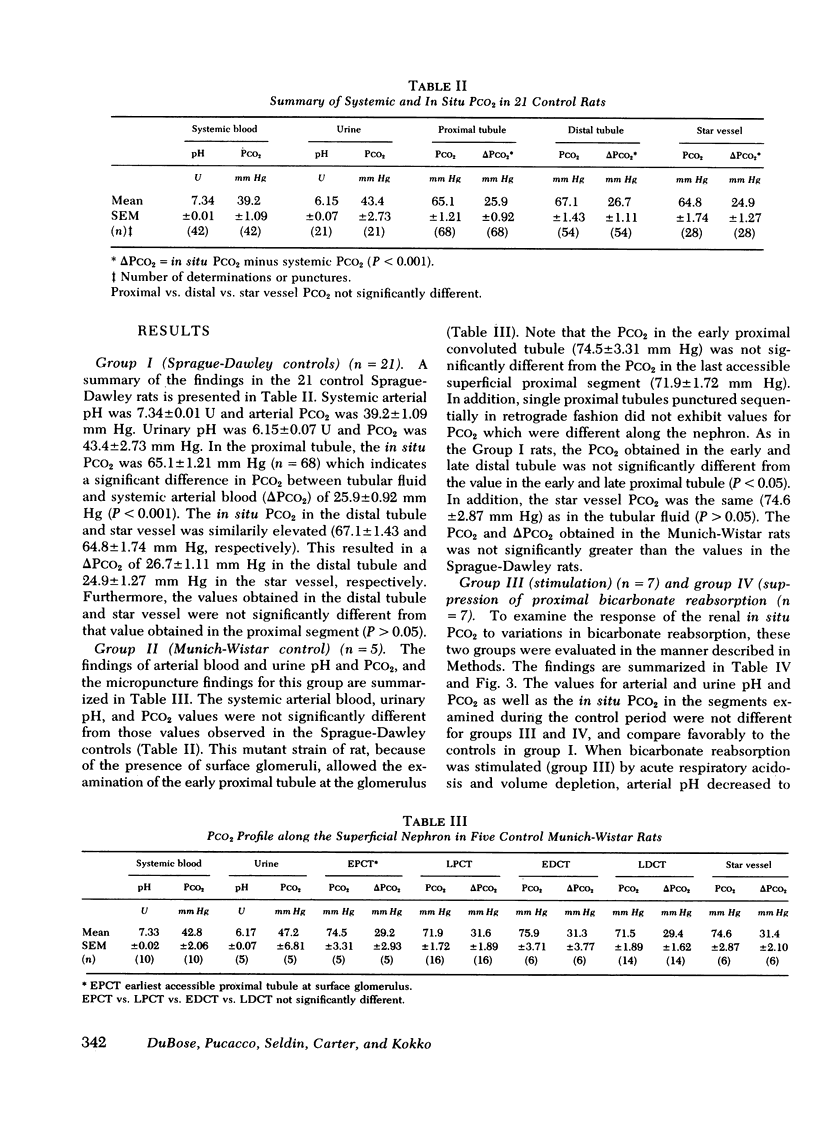
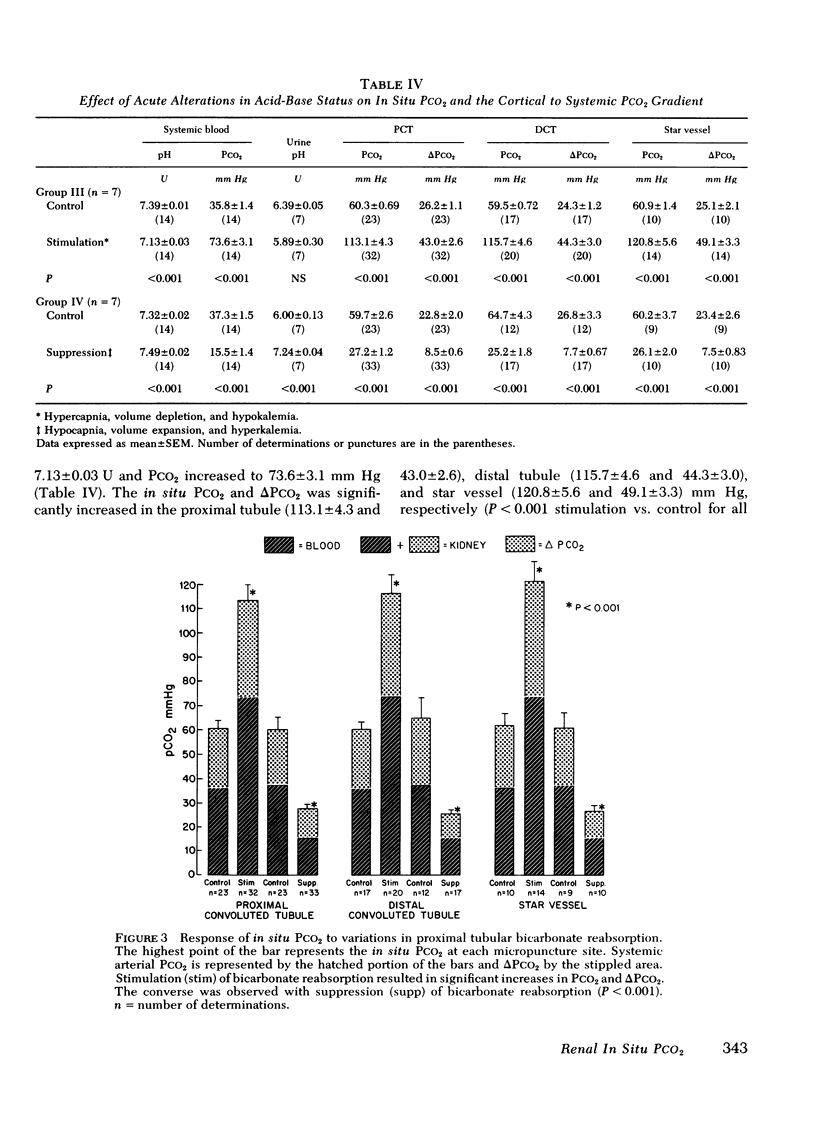
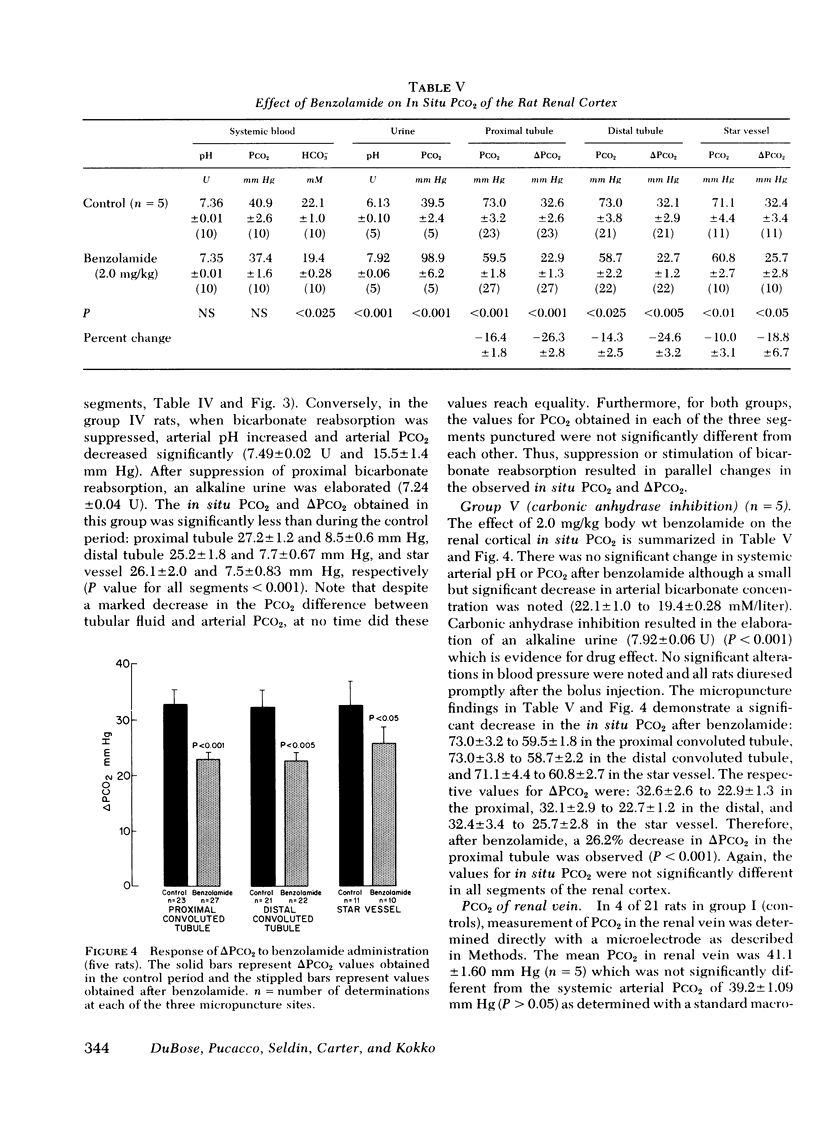
Abstract
The mechanism by which the kidney reabsorbs sodium bicarbonate could be a result of (a) H+ secretion, (b) direct HCO3- reabsorption, or (c) a combination of both processes. Most of the studies which have supported the H+ secretory theory have involved the assumption that tubular fluid and arterial PCO2 were equal. We have utilized a new PCO2 microelectrode to directly determine in situ PCO2 of tubular fluid and stellate vessel blood in the cortex of the rat kidney during control conditions and after alterations in acid-base status. In 21 control rats, proximal tubular fluid PCO2 exceeded systemic arterial PCO2 (deltaCO2) by 25.9 +/- 0.92 mm Hg (P less than 0.001). The values obtained for both distal tubular fluid and stellate vessel blood were not significantly different from proximal tubular PCO2. Evaluation of PCO2 in the proximal tubules of Munich-Wistar rats did not reveal evidence for a declining profile for PCO2 along the length of the nephron. When proximal bicarbonate reabsorption was increased or decreased acutely by alterations in acid-base status, deltaPCO2 changed in paralle. Furthermore, benzolamide administration significantly reduced deltaPCO2. We conclude: (a) that the PCO2 in tubular fluid is significantly greater than systemic arterial PCO2, (b) that there is no tendency for the observed PCO2 to fall along the proximal tubule, (c) the mean PCO2 in the proximal and distal tubules as well as the stellate vessle is not significantly different, thereby rendering the concept of a "diffusion barrier" for CO2 in the proximal tubule unlikely, and (d) the level of renal cortical PCO2 appears to vary directly with the magnitude of bicarbonate reabsorption.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arruda J. A., Nascimento L., Kumar S. K., Kurtzman N. A. Factors influencing the formation of urinary carbon dioxide tension. Kidney Int. 1977 May;11(5):307–317. doi: 10.1038/ki.1977.48. [DOI] [PubMed] [Google Scholar]
- Arruda J. A., Nascimento L., Mehta P. K., Rademacher D. R., Sehy J. T., Westenfelder C., Kurtzman N. A. The critical importance of urinary concentrating ability in the generation of urinary carbon dioxide tension. J Clin Invest. 1977 Oct;60(4):922–935. doi: 10.1172/JCI108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caflisch C. R., Carter N. W. A micro pCO2 electrode. Anal Biochem. 1974 Jul;60(1):252–257. doi: 10.1016/0003-2697(74)90151-1. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Seldin D. W., Kokko J. P. Segmental chloride reabsorption in the rat nephron as a function of load. Am J Physiol. 1978 Feb;234(2):F97–105. doi: 10.1152/ajprenal.1978.234.2.F97. [DOI] [PubMed] [Google Scholar]
- Filho E. M., Malnic G. H in cortical peritubular capillaries of rat kidney. Pflugers Arch. 1976 Jun 22;363(3):211–217. doi: 10.1007/BF00594603. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G. Studies on the mechanism of tubular acidification. Physiologist. 1976 Nov;19(4):511–524. [PubMed] [Google Scholar]
- Karlmark B., Danielson B. G. Titratable acid, PCO2, bicarbonate and ammonium ions along the rat proximal tubule. Acta Physiol Scand. 1974 Jun;91(2):243–258. doi: 10.1111/j.1748-1716.1974.tb05681.x. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr The influence of the carbonic anhydrase inhibitor, benzolamide (CL-11,366), on the reabsorption of chloride, sodium, and bicarbonate in the proximal tubule of the rat. J Clin Invest. 1972 Feb;51(2):294–306. doi: 10.1172/JCI106814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Malnic G., Giebisch G. Symposium on acid-base homeostasis. Mechanism of renal hydrogenion secretion. Kidney Int. 1972 May;1(5):280–296. doi: 10.1038/ki.1972.41. [DOI] [PubMed] [Google Scholar]
- Malnic G., Steinmetz P. R. Transport processes in urinary acidification. Kidney Int. 1976 Feb;9(2):172–188. doi: 10.1038/ki.1976.19. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Chemistry of the renal reabsorption of bicarbonate. Can J Physiol Pharmacol. 1974 Dec;52(6):1041–1050. doi: 10.1139/y74-138. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Pucacco L. R., Carter N. W. A glass-membrane pH microelectrode. Anal Biochem. 1976 Jun;73(2):501–512. doi: 10.1016/0003-2697(76)90200-1. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Zurbach P. E. Re-evaluation of microelectrode methodology for the in vitro determination of pH and bicarbonate concentration. Kidney Int. 1974 Aug;6(2):81–91. doi: 10.1038/ki.1974.83. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Finn J. T., Vaughan G., Steinmetz P. R. Distribution of metabolic CO2 and the transported ion species in acidification by turtle bladder. Am J Physiol. 1974 Feb;226(2):283–289. doi: 10.1152/ajplegacy.1974.226.2.283. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. CO2 requirements for H+ secretion by the isolated turtle bladder. Am J Physiol. 1971 Jun;220(6):2051–2057. doi: 10.1152/ajplegacy.1971.220.6.2051. [DOI] [PubMed] [Google Scholar]
- Sohtell M., Karlmark B. In vivo micropuncture PCO2 measurements. Pflugers Arch. 1976 May 12;363(2):179–180. doi: 10.1007/BF01062288. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]


