Abstract
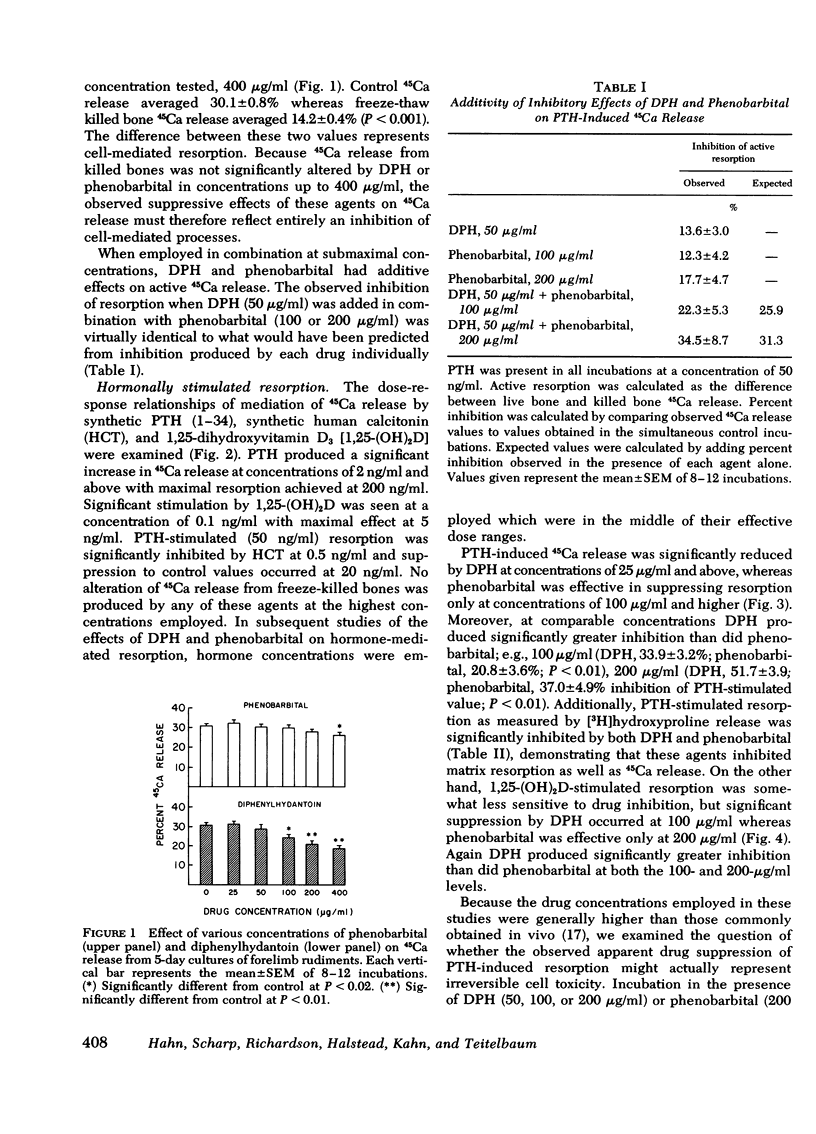
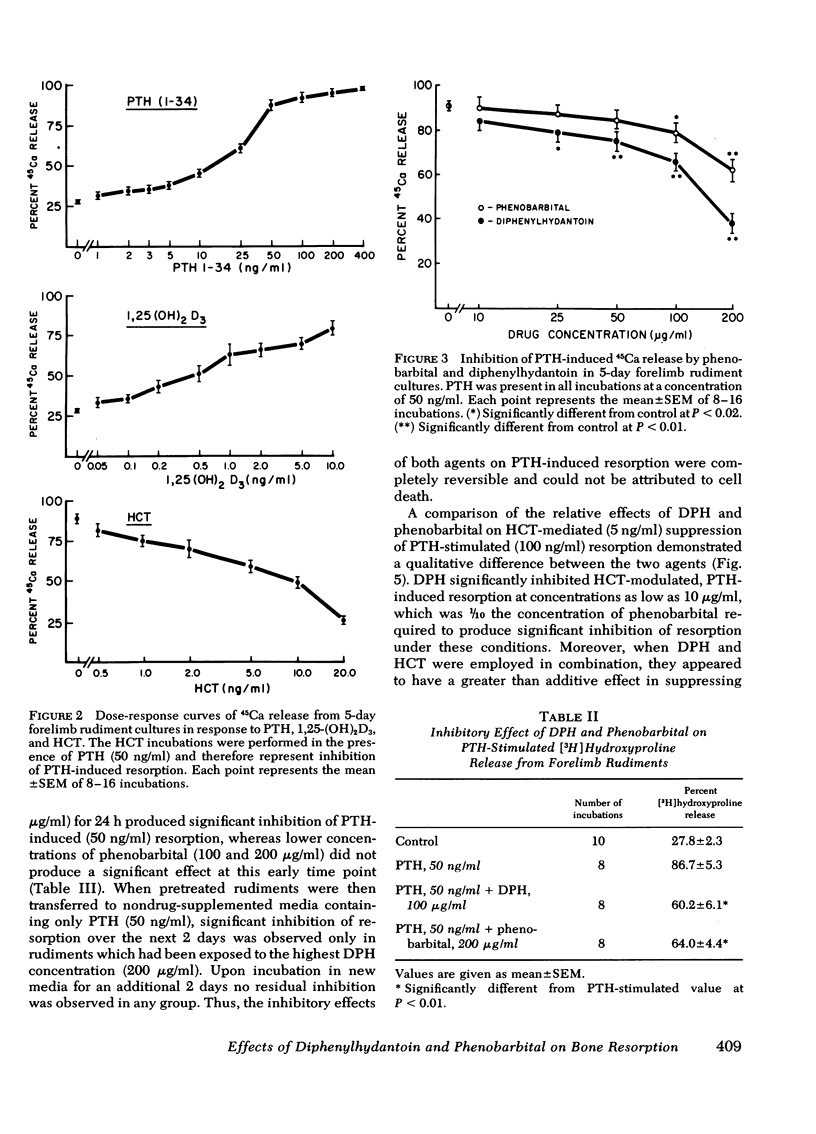
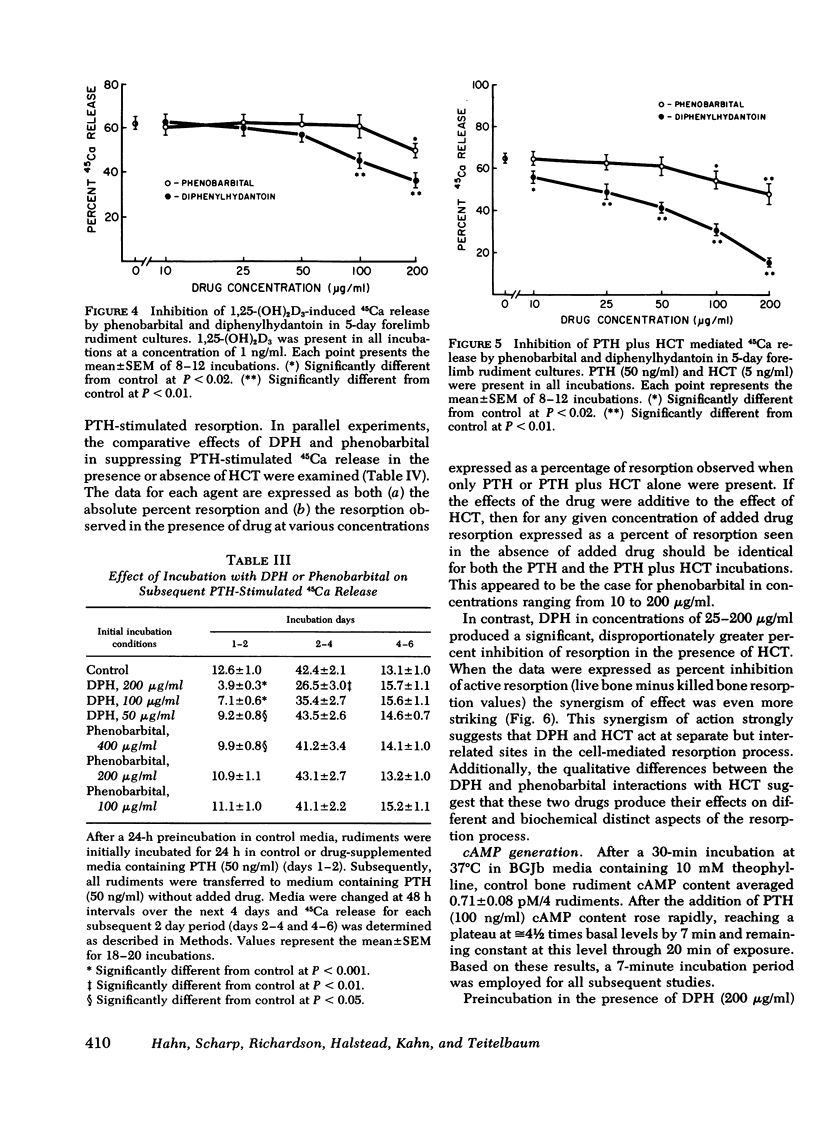
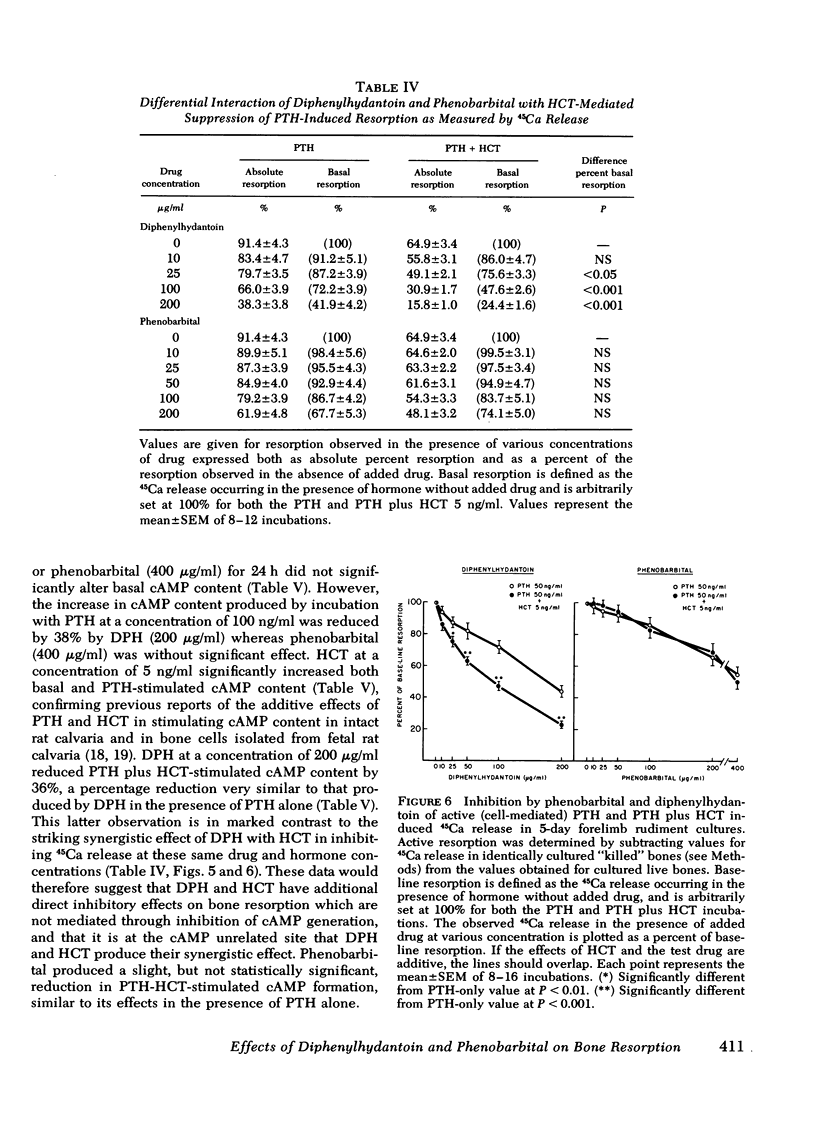
Chronic administration of high doses of anticonvulsant drugs frequently produces classic osteomalacia with bone histologic changes characteristic of increased parathyroid hormone (PTH) effect in man. However, several reports have documented defects in calcified tissue metabolism suggestive of an end-organ resistance to PTH after chronic anticonvulsant drug therapy. To examine the direct action of anticonvulsant drugs on bone resorption, we investigated the effects of diphenylhydantoin (phenytoin) (DPH) (100-200 μg/ml) and phenobarbital (10-400 μg/ml) on basal and hormonally mediated resorption 5-day cultures of fetal rat forelimb rudiments. In this system both drugs significantly inhibited basal and PTH-stimulated 45Ca and [3H]hydroxyproline release, as well as 1,25-dihydroxyvitamin D3-stimulated 45Ca release. The effects of DPH and phenobarbital were additive, with DPH exhibiting a several-fold more potent inhibitory effect than phenobarbital. Whereas DPH exhibited a striking synergism with the inhibitory effects of human calcitonin (HCT) on PTH-induced resorption, the effect of phenobarbital was merely additive to that of HCT. PTH and PTH plus HCT-induced increases in bone cyclic AMP (cAMP) content were significantly inhibited by DPH but not by phenobarbital. However, in contrast to effects on 45Ca release, DPH inhibition of cAMP generation was not accentuated in the presence of HCT. It is concluded that: (a) both DPH and phenobarbital can directly inhibit basal and hormonally stimulated bone resorption, with DPH being much more potent in this regard; (b) DPH appears to inhibit bone resorption via a cAMP-independent mechanism and has an additional suppressive effect on PTH-induced cAMP generation; and (c) the synergistic interaction of DPH and HCT in inhibiting 45Ca release occurs at a site independent of cAMP generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGERS J. D., GWATKIN R. B., HEYNER S. Growth of embryonic avian and mammalian tibiae on a relatively simple chemically defined medium. Exp Cell Res. 1961 Oct;25:41–58. doi: 10.1016/0014-4827(61)90305-6. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Jr, Gilbert H. R., Avioli L. V. Intestinal calcium transport: the role of sodium. Science. 1972 Apr 14;176(4031):168–170. doi: 10.1126/science.176.4031.168. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Reynaert J., Claes J. H., Lissens W., De Moor P. The effect of anticonvulsant therapy on serum levels of 25-hydroxy-vitamin D, calcium, and parathyroid hormone. J Clin Endocrinol Metab. 1975 Dec;41(06):1130–1135. doi: 10.1210/jcem-41-6-1130. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. The effect of parathyroid hormone on the concentration of adenosine 3',5'-monophosphate in skeletal tissue in vitro. J Biol Chem. 1970 Apr 10;245(7):1520–1526. [PubMed] [Google Scholar]
- Dent C. E., Richens A., Rowe D. J., Stamp T. C. Osteomalacia with long-term anticonvulsant therapy in epilepsy. Br Med J. 1970 Oct 10;4(5727):69–72. doi: 10.1136/bmj.4.5727.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziak R., Stern P. H. Calcium transport in isolated bone cells. III. Effects of parathyroid hormone and cyclic 3',5'-AMP. Endocrinology. 1975 Nov;97(5):1281–1287. doi: 10.1210/endo-97-5-1281. [DOI] [PubMed] [Google Scholar]
- Dziak R., Stern P. H. Responses of fetal rat bone cells and bone organ cultures to the ionophore, A23187. Calcif Tissue Res. 1976 Dec 22;22(2):137–147. doi: 10.1007/BF02010353. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Kinscherf D. A. Phenytoin: effects on calcium flux and cyclic nucleotides. Epilepsia. 1977 Sep;18(3):331–336. doi: 10.1111/j.1528-1157.1977.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Hahn T. J., Birge S. J., Scharp C. R., Avioli L. V. Phenobarbital-induced alterations in vitamin D metabolism. J Clin Invest. 1972 Apr;51(4):741–748. doi: 10.1172/JCI106868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harell A., Binderman I., Rodan G. A. The effect of calcium concentration on calcium uptake by bone cells treated with thyrocalcitonin (TCT) hormone. Endocrinology. 1973 Feb;92(2):550–555. doi: 10.1210/endo-92-2-550. [DOI] [PubMed] [Google Scholar]
- Harris M., Goldhaber P. Root abnormalities in epileptics and the inhibition of parathyroid hormone induced bone resorption by diphenyl hydantoin in tissue culture. Arch Oral Biol. 1974 Nov;19(11):981–984. doi: 10.1016/0003-9969(74)90083-1. [DOI] [PubMed] [Google Scholar]
- Harris M., Jenkins M. V., Wills M. R. Phenytoin inhibition of parathyroid hormone induced bone resorption in vitro. Br J Pharmacol. 1974 Mar;50(3):405–408. doi: 10.1111/j.1476-5381.1974.tb09616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heersche J. N., Marcus R., Aurbach G. D. Calcitonin and the formation of 3',5'-AMP in bone and kidney. Endocrinology. 1974 Jan;94(1):241–247. doi: 10.1210/endo-94-1-241. [DOI] [PubMed] [Google Scholar]
- Hunter J., Maxwell J. D., Stewart D. A., Parsons V., Williams R. Altered calcium metabolism in epileptic children on anticonvulsants. Br Med J. 1971 Oct 23;4(5781):202–204. doi: 10.1136/bmj.4.5781.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey J. L., Wright D. R., Tashjian A. H., Jr Bone resorption in organ culture: inhibition by the divalent cation ionophores A23187 and X-537A. J Clin Invest. 1976 Dec;58(6):1327–1338. doi: 10.1172/JCI108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. V., Harris M., Wills M. R. The effect of phenytoin on parathyroid extract and 25-hydroxycholecalciferol-induced bone resorption: adenosine 3, 5 cyclic monophosphate production. Calcif Tissue Res. 1974;16(2):163–167. doi: 10.1007/BF02008223. [DOI] [PubMed] [Google Scholar]
- Jensen B. N., Grynderup V. Studies on the metabolism of phenytoin. Epilepsia. 1966 Sep;7(3):238–245. doi: 10.1111/j.1528-1157.1966.tb03802.x. [DOI] [PubMed] [Google Scholar]
- Kattan K. R. Calvarial thickening after Dilantin medication. Am J Roentgenol Radium Ther Nucl Med. 1970 Sep;110(1):102–105. doi: 10.2214/ajr.110.1.102. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Raisz L. G. Role of adenosine-3',5'-monophosphate in the hormonal regulation of bone resorption: studies with cultured fetal bone. Endocrinology. 1971 Sep;89(3):818–826. doi: 10.1210/endo-89-3-818. [DOI] [PubMed] [Google Scholar]
- Koch H. U., Kraft D., von Herrath D., Schaefer K. Influence of diphenylhydantoin and phenobarbital on intestinal calcium transport in the rat. Epilepsia. 1972 Dec;13(6):829–834. doi: 10.1111/j.1528-1157.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Lund L., Berlin A., Lunde K. M. Plasma protein binding of diphenylhydantoin in patients with epilepsy. Agreement between the unbound fraction in plasma and the concentration in the cerebrospinal fluid. Clin Pharmacol Ther. 1972 Mar-Apr;13(2):196–200. doi: 10.1002/cpt1972132196. [DOI] [PubMed] [Google Scholar]
- Martin D. L., DeLuca H. F. Influence of sodium on calcium transport by the rat small intestine. Am J Physiol. 1969 Jun;216(6):1351–1359. doi: 10.1152/ajplegacy.1969.216.6.1351. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Dowling I. Failure of 1, 25 dihydroxycholecalciferol (1, 25-(OH) 2-D3) to modify cyclic AMP levels in parathyroid hormone-treated and untreated bone cells. Endocr Res Commun. 1976;3(2):157–166. doi: 10.3109/07435807609052930. [DOI] [PubMed] [Google Scholar]
- Pincus J. H. Diphenylhydantoin and ion flux in lobster nerve. Arch Neurol. 1972 Jan;26(1):4–10. doi: 10.1001/archneur.1972.00490070022003. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Richens A., Rowe D. J. Disturbance of calcium metabolism by anticonvulsant drugs. Br Med J. 1970 Oct 10;4(5727):73–76. doi: 10.1136/bmj.4.5727.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Rodan G. A. The effect of parathyroid hormone and thyrocalcitonin on the accumulation of cyclic adenosine 3':5'-monophosphate in freshly isolated bone cells. J Biol Chem. 1974 May 25;249(10):3068–3074. [PubMed] [Google Scholar]
- Seltzer J. L., Welgus H. G., Jeffrey J. J., Eisen A. Z. The function of Ca+ in the action of mammalian collagenases. Arch Biochem Biophys. 1976 Mar;173(1):355–361. doi: 10.1016/0003-9861(76)90270-8. [DOI] [PubMed] [Google Scholar]
- Silver J., Neale G., Thompson G. R. Effect of phenobarbitone treatment on vitamin D metabolism in mammals. Clin Sci Mol Med. 1974 Apr;46(4):433–448. doi: 10.1042/cs0460433. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Johnston C. C., Jr Hormonal responsiveness of adenylate cyclase activity from separate bone cells. Endocrinology. 1974 Jul;95(1):130–139. doi: 10.1210/endo-95-1-130. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman K. G., Jubiz W., Sannella J. J., Madsen J. A., Belsey R. E., Goldsmith R. S., Freston J. W. Osteomalacia associated with anticonvulsant drug therapy in mentally retarded children. Pediatrics. 1975 Jul;56(1):45–50. [PubMed] [Google Scholar]
- Wong G. L., Luben R. A., Cohn D. V. 1,25-dihydroxycholecalciferol and parathormone: effects on isolated osteoclast-like and osteoblast-like cells. Science. 1977 Aug 12;197(4304):663–665. doi: 10.1126/science.195343. [DOI] [PubMed] [Google Scholar]


