Abstract
Lysophosphoglycerides, products of membrane phospholipid catabolism known to influence membrane function in several systems, appeared in the effluents of anoxic isolated rabbit hearts perfused at low flow and accumulated in perfused hearts and myocardium rendered ischemic in situ. Comparable concentrations of lysophosphoglycerides bound to albumin markedly and reversibly altered action potentials of isolated canine Purkinje fibers in vitro. Changes induced included diminution of the maximum diastolic potential, peak dV/dt of phase zero, amplitude, and action potential duration--alterations resembling those seen in ischemic myocardium in vivo. These electrophysiological alterations are compatible with changes implicated in predisposing to dysrhythmia dependent on reentry, a phenomenon potentiated by the presence of zones of decreased conduction. Thus, accumulation of lysophosphoglycerides induced by ischemia may contribute to the genesis of malignant dysrhythmia early after its onset.
Full text
PDF
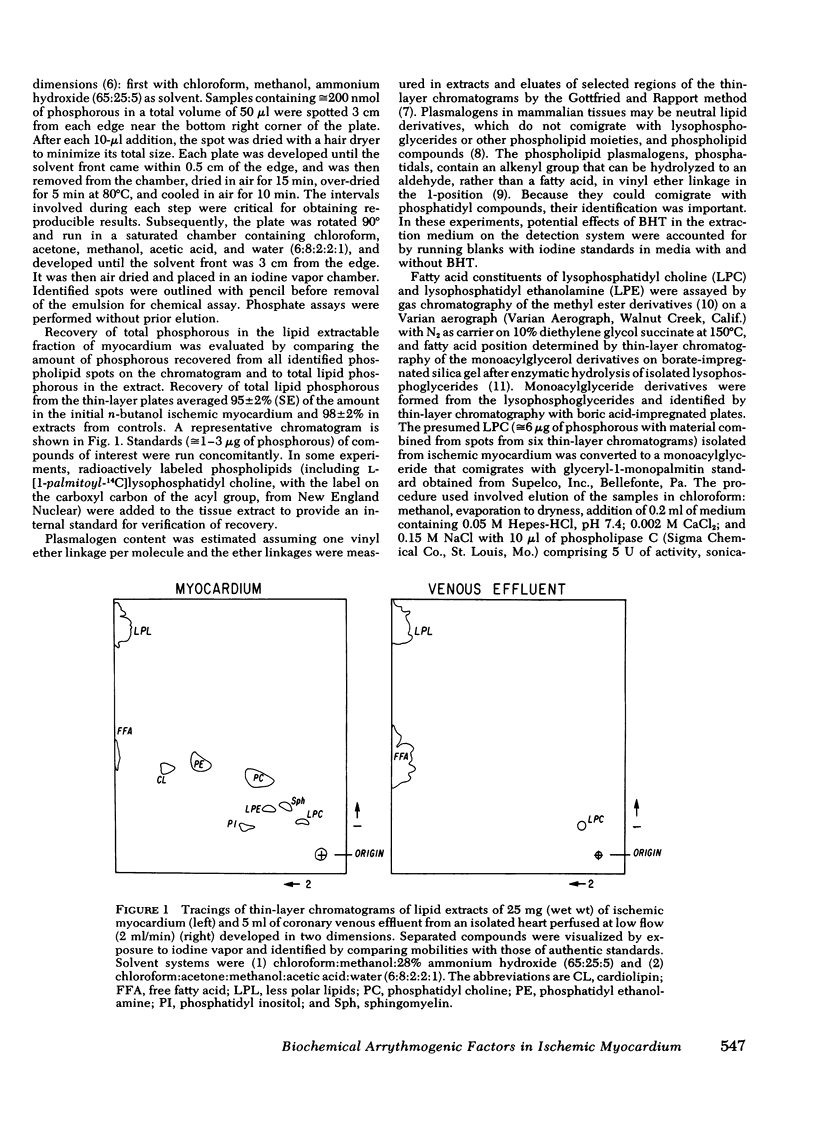
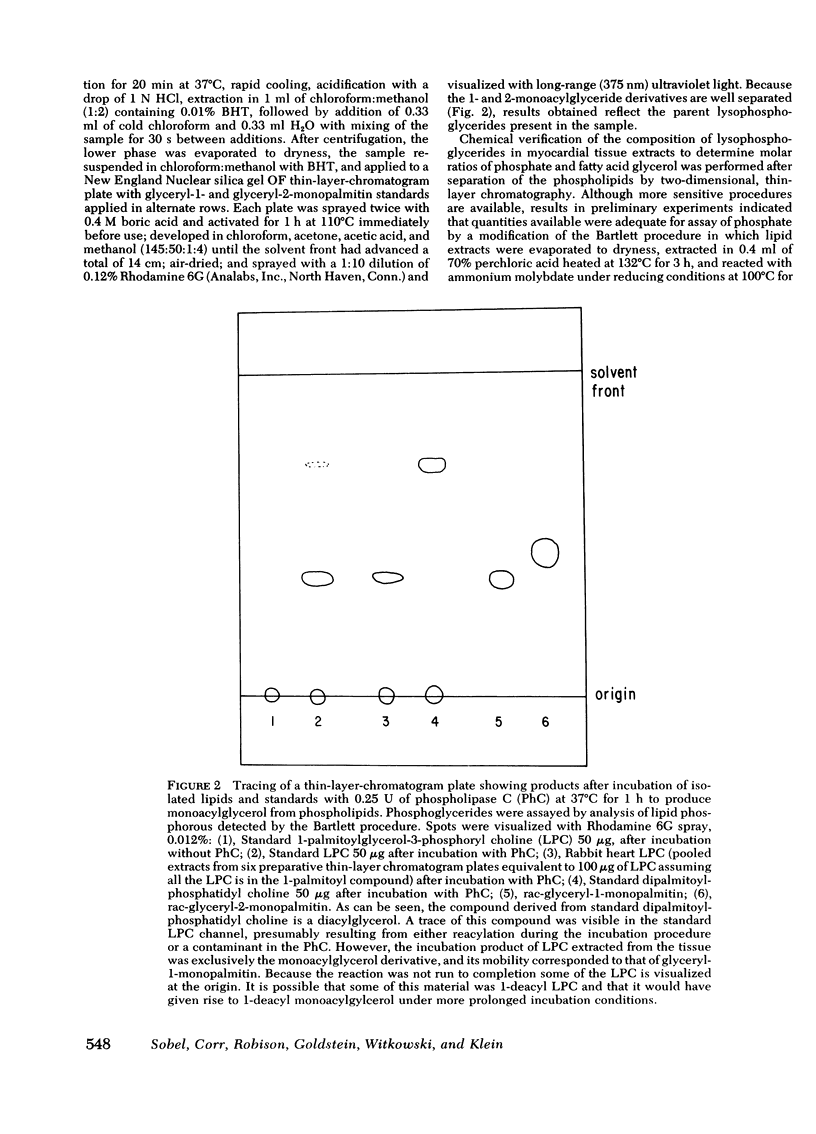

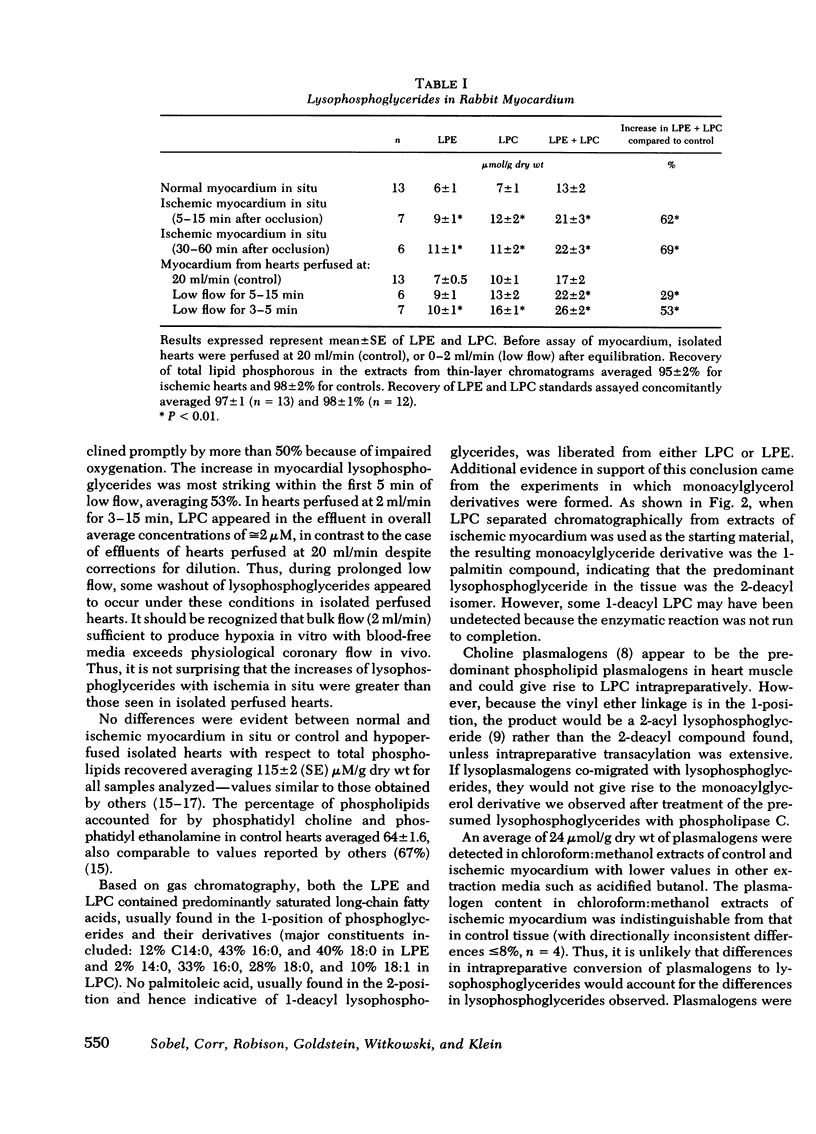
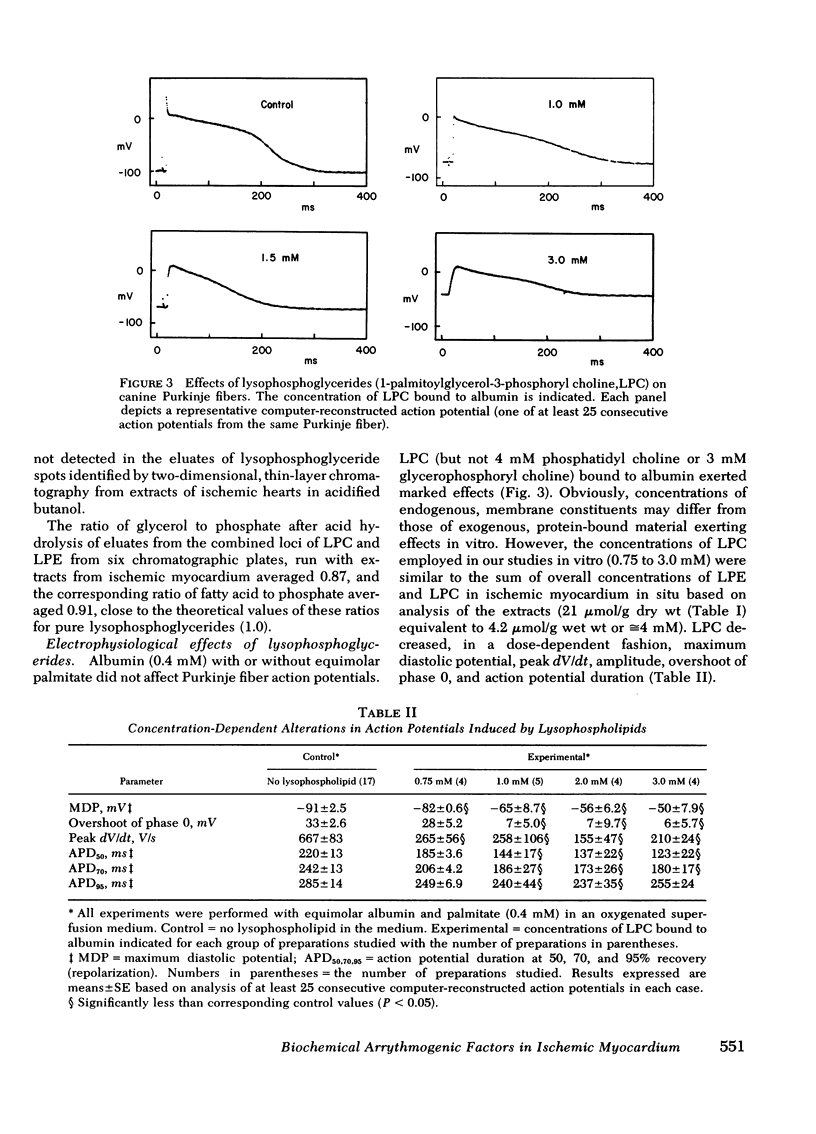


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigger J. T., Jr, Dresdale F. J., Heissenbuttel R. H., Weld F. M., Wit A. L. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis. 1977 Jan-Feb;19(4):255–300. doi: 10.1016/0033-0620(77)90005-6. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Witkowski F. X., Sobel B. E. Mechanisms contributing to malignant dysrhythmias induced by ischemia in the cat. J Clin Invest. 1978 Jan;61(1):109–119. doi: 10.1172/JCI108908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of "ischemic" blood on transmembrane potentials of normal porcine ventricular myocardium. Circulation. 1977 Mar;55(3):455–462. doi: 10.1161/01.cir.55.3.455. [DOI] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation. 1977 Aug;56(2):217–224. doi: 10.1161/01.cir.56.2.217. [DOI] [PubMed] [Google Scholar]
- GOTTFRIED E. L., RAPPORT M. M. The biochemistry of plasmalogens. I. Isolation and characterization of phosphatidal choline, a pure native plasmalogen. J Biol Chem. 1962 Feb;237:329–333. [PubMed] [Google Scholar]
- Gazitt Y., Ohad I., Loyter A. Changes in phosoholipid susceptibility toward phospholipases induced by ATP depletion in avian and amphibian erythrocyte membranes. Biochim Biophys Acta. 1975 Feb 28;382(1):65–72. doi: 10.1016/0005-2736(75)90373-9. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Oskarsdóttir G. Changes in fatty acid composition of cardiac lipids accompanying myocardial necrosis. Recent Adv Stud Cardiac Struct Metab. 1975;6:193–203. [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Needleman P., Marshall G. R., Sobel B. E. Hormone interactions in the isolated rabbit heart. Synthesis and coronary vasomotor effects of prostaglandins, angiotensin, and bradykinin. Circ Res. 1975 Dec;37(6):802–808. doi: 10.1161/01.res.37.6.802. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Mavis R. D., Vagelos P. R. The specific acylation of glycerol 3-phosphate to monoacylglycerol 3-phosphate in Escherichia coli. Evidence for a single enzyme conferring this specificity. J Biol Chem. 1970 Dec 10;245(23):6442–6448. [PubMed] [Google Scholar]
- Silbert D. F., Ulbright T. M., Honegger J. L. Utilization of exogenous fatty acids for complex lipid biosynthesis and its effect on de novo fatty acid formation in Escherichia coli K-12. Biochemistry. 1973 Jan 2;12(1):164–171. doi: 10.1021/bi00725a027. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]


