Abstract
Anthropogenic drivers of environmental change often have multiple effects, including changes in biodiversity, species composition, and ecosystem functioning. It remains unknown whether such shifts in biodiversity and species composition may, themselves, be major contributors to the total, long-term impacts of anthropogenic drivers on ecosystem functioning. Moreover, although numerous experiments have shown that random losses of species impact the functioning of ecosystems, human-caused losses of biodiversity are rarely random. Here we use results from long-term grassland field experiments to test for direct effects of chronic nutrient enrichment on ecosystem productivity, and for indirect effects of enrichment on productivity mediated by resultant species losses. We found that ecosystem productivity decreased through time most in plots that lost the most species. Chronic nitrogen addition also led to the nonrandom loss of initially dominant native perennial C4 grasses. This loss of dominant plant species was associated with twice as great a loss of productivity per lost species than occurred with random species loss in a nearby biodiversity experiment. Thus, although chronic nitrogen enrichment initially increased productivity, it also led to loss of plant species, including initially dominant species, which then caused substantial diminishing returns from nitrogen fertilization. In contrast, elevated CO2 did not decrease grassland plant diversity, and it consistently promoted productivity over time. Our results support the hypothesis that the long-term impacts of anthropogenic drivers of environmental change on ecosystem functioning can strongly depend on how such drivers gradually decrease biodiversity and restructure communities.
Keywords: biogeochemistry, community ecology
Humans disturbances such as land clearing, intensive grazing, nutrient addition, pesticide use, commercial fishing, and release of greenhouse gasses are impacting the species compositions, biodiversity, and functioning of terrestrial and marine ecosystems worldwide (1). Although there are well-described effects of many of these disturbances on ecosystems, the underlying causes are often poorly understood. For instance, nitrogen (N) deposition simultaneously increases the availability of a major limiting nutrient, accelerates the leaching loss of base cations that may also be limiting, favors some plant species over others, causes the loss of plant diversity, and increases primary productivity (2–7). Is the path of causation a simple direct path from elevated N deposition to each of these responses, or are there one or more intermediaries that, in a chain of causation, influence the long-term effects of disturbances on ecosystem functioning? Here we consider whether changes in biodiversity might mediate the long-term effects of anthropogenic disturbances on ecosystem functioning.
We also consider whether nonrandom losses of species associated with human disturbances have a quantitatively different impact on ecosystem functioning than do random species losses. There is considerable theoretical and experimental evidence that random species losses can substantially decrease productivity in terrestrial, freshwater, and marine ecosystems (8–15). This has raised concerns that contemporary biodiversity declines might alter ecosystem functioning and decrease the provision of several ecosystem services (16–18). Some ecologists, however, have questioned the relevance of these results for conservation (19, 20). Species losses are often nonrandom, and nonrandom species losses may impact ecosystem functioning more, less, or the same amount as random species losses, depending in part on which species are lost (21–23). For example, one previous study found that experimentally removing rare species had little effect on community productivity (22). Similarly, in another study, productivity remained unchanged as the most productive species became increasingly dominant across treatments that had progressively lower species evenness (21). These results suggest that nonrandom biodiversity declines might have less impact on productivity if the most productive species persist and become increasingly dominant. Although these studies considered plausible scenarios, an important next step is to consider the consequences of nonrandom species losses that result from common drivers of contemporary biodiversity declines, such as nutrient enrichment, which may or may not result in the persistence and dominance of the most productive species.
It seems plausible that global change drivers might directly influence productivity much more than they indirectly influence productivity through their effects on biodiversity (19, 20, 24). For example, biodiversity effects might be negligible if nutrient enrichment increases productivity by altering physiological responses and reducing resource limitation much more than it decreases productivity by driving nonrandom biodiversity declines (19, 20, 24, 25). However, previous studies have not determined the extent to which global environmental changes indirectly influence ecosystem functioning by nonrandomly changing biodiversity (19, 20, 24). Thus, it remains unclear whether it is necessary to account for the effects of global environmental changes on biodiversity when determining their long-term impacts on ecosystem functioning and services (19, 20, 24). It has been hypothesized that global change drivers will initially impact ecosystems by altering physiological responses, and later impact ecosystems by changing plant species dominance and richness (25). Thus, the indirect effects of global change drivers on productivity via changes in biodiversity, which we consider here, may only be evident in long-term studies.
Here we first explore results from the long-term Nitrogen Enrichment Experiment by analyzing temporal trends in the effects of N enrichment on the productivity, plant diversity, and species compositions of naturally assembled grasslands. Our long-term N addition experiment (7, 26, 27) manipulated N enrichment (0, 10, 20, 34, 54, 95, 170, or 270 kg N ha−1 y−1) and measured aboveground peak biomass, as well as the number and abundances of plant species in each plot from 1982 to 2008 in naturally assembled grasslands at the Cedar Creek Ecosystem Science Reserve in central Minnesota, United States (261 plots, each 4 m by 4 m) (Methods). These data allow us to determine how nonrandom species losses caused by N addition influenced productivity and to compare the diversity-dependence of productivity in this experiment with that observed in the Cedar Creek Biodiversity Experiment (14, 28), which considered random species losses. Next, we similarly use data from the BioCON (Biodiversity, CO2, and N) experiment located at the same reserve to quantify the extent to which N enrichment and elevated CO2 influence productivity by nonrandomly changing grassland plant diversity. The BioCON experiment (5, 6, 13, 29) crossed plant diversity (1, 4, 9, or 16 species), CO2 (elevated or ambient), and N (enriched or ambient) treatments and measured productivity and the number of remaining plant species in each plot from 1998 to 2011 (Methods). To quantify the net effects (also known as “the effects”) of N and CO2 on productivity we fit linear mixed effects models that accounted for the split-plot experimental treatment design and repeated measurements (Methods). We also used structural equation modeling to disentangle the direct and indirect effects of both N enrichment and elevated CO2 on productivity (Fig. S1) (Methods).
Results and Discussion
Nitrogen Enrichment Experiment.
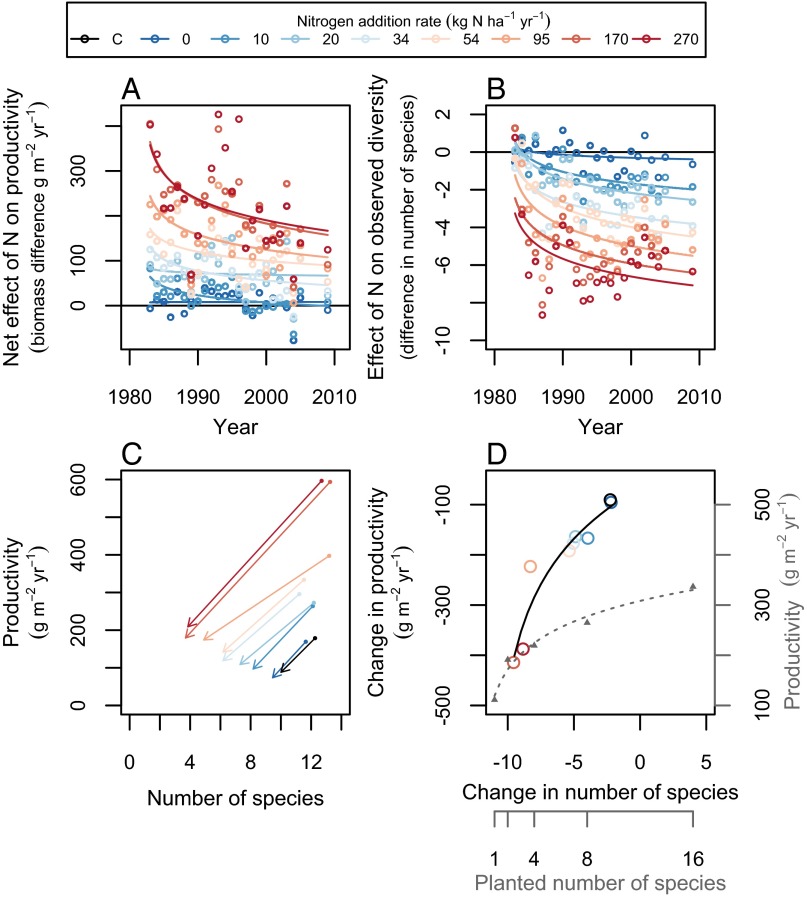
Although N enrichment initially increased plant productivity, the magnitude of this effect declined through time in natural grasslands. Specifically, nutrient enrichment promoted productivity, but this effect substantially diminished over time, especially in the plots that received the most fertilizer (Fig. 1A). Nutrient enrichment also decreased the number of plant species, and this effect became increasingly negative over time at all rates of N addition (Fig. 1B). Thus, nutrient enrichment resulted in more species losses than species gains, especially in later years. Moreover, species losses were nonrandom, with initially dominant native perennial C4 grasses, particularly Schizachyrium scoparium, becoming less dominant and then lost, and nonnative perennial C3 grasses, particularly Elymus repens (formerly Agropyron/Elytrigia repens) becoming increasingly dominant (7, 27, 30). For example, at high rates of N addition in field C, plant communities shifted from a high-diversity native-dominated state to a self-reinforcing low-diversity state with few or no native species (31). Nine native species were particularly susceptible to becoming locally extinct under chronic nutrient enrichment, including initially dominant (e.g., S. scoparium) and initially rare (e.g., Liatris aspera) species (26).
Fig. 1.
Effects of N addition on productivity and plant diversity in natural grasslands. (A) Nutrient enrichment promoted productivity (F7,176 = 54.5, P < 2.2 × 10−16), but this effect substantially diminished over time (F1,176 = 36.9, P = 7.48 × 10−9), especially in the plots that received the most fertilizer (N × Year: F7,176 = 2.8, P = 0.0096). (B) Nutrient enrichment also decreased the number of species (F7,176 = 72.4, P < 2.2 × 10−16), and this effect became increasingly negative over time (F1,176 = 92.1, P < 2.2 × 10−16) at all rates of N addition (N × Year: F7,176 = 1.6, P = 0.13). (C and D) Declines in the number of species were positively associated with declines in productivity. That is, productivity decreased most in plots that lost the most species over time (F1,7 = 42.9, P = 0.00032, R2 = 0.86) (D). Differences in biomass (A) or number of species (B) were quantified between enriched and control plots, with positive biomass differences indicating that enriched plots were more productive, and with negative species differences indicating that enriched plots had fewer species, than control plots. Arrows in C point from values observed during 1982, the first year of N addition, toward values observed during 2008, the most recent year that all fields were sampled. Changes in D were quantified over the same time interval. In D, for comparison, we also show the relationship between planted species richness and productivity in a nearby biodiversity experiment during 2008 (gray triangles and dashed line). Both lines in D are linear fits of the response on the ln-transformed predictor. Treatments C (control) and 0 differ in that non-N nutrients were added to the latter but not the former.
Productivity decreased most in plots that lost the most species over time (Fig. 1D). Specifically, productivity decreased by ∼40 g m−2 y−1 for every species loss under nutrient enrichment (Fig. 1D, slope = 39). High rates of N addition increased productivity by as much as 400 g m−2 y−1 early on, but over a period of 25 y the productivity increase fell to less than 200 g m−2 y−1 as more than half the plant species were lost from these plots (Fig. 1 A and C).
The loss of dominant plant species led to a much greater loss of productivity than does the random loss of species. In particular, the decrease in productivity associated with nonrandom species losses resulting from nutrient enrichment was greater than the decrease in productivity observed under random species losses in a nearby biodiversity experiment (compare the two curves in Fig. 1D). This suggests that ecologically realistic nonrandom species losses could decrease productivity more than the random species losses considered by many biodiversity experiments. For example, this could occur when initially dominant species are lost (22), such as S. scoparium in our study (7, 27), as previously predicted (25, 32).
BioCON Experiment.
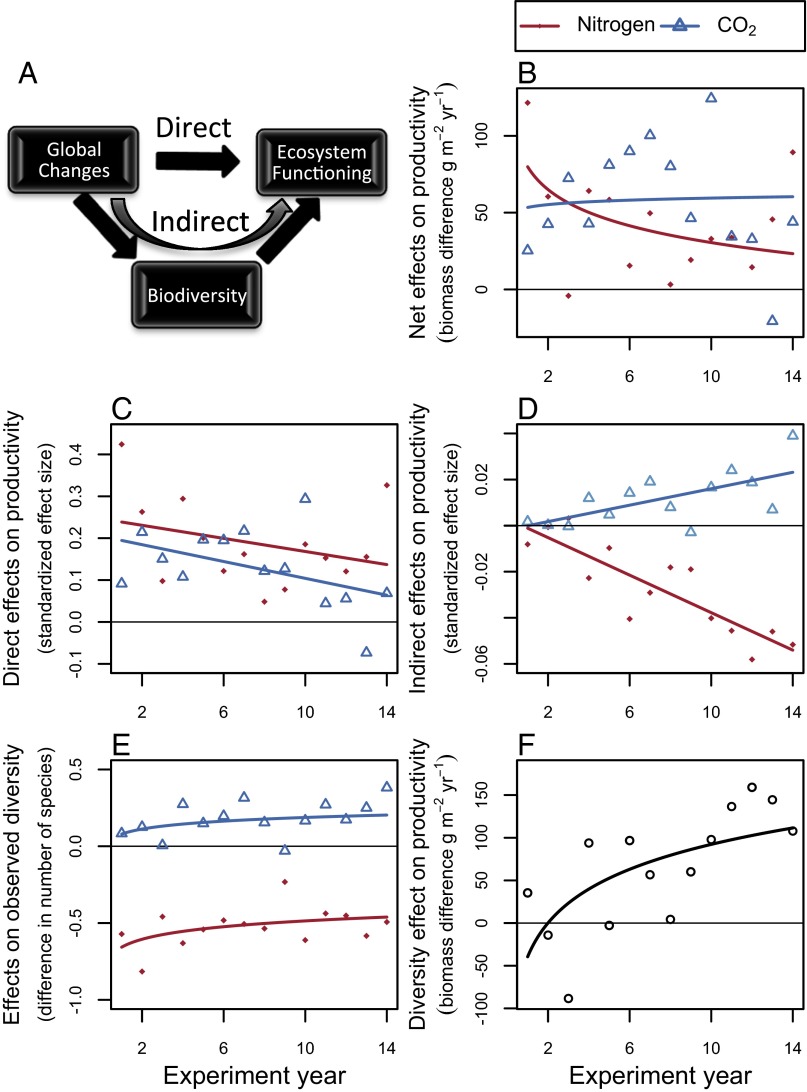
The magnitude of the initially positive net effect of N enrichment on productivity also substantially decreased over time in the BioCON experiment (Fig. 2B and Table S1). In contrast, the net effect of elevated CO2 on productivity did not have a detectable temporal decline (Fig. 2B and Table S1).
Fig. 2.
(A) Global environmental changes may indirectly influence ecosystem functioning by changing biodiversity. (B–F) Results from the BioCON experiment. Net effects on grassland plant productivity (B), which can be split into direct (C) and indirect (D) components, which can subsequently be split into effects of N and CO2 on observed diversity (E) and the effect of planted diversity on productivity (F). The positive effect of N enrichment on productivity diminished over time (P = 0.0188) (B), partly because N enrichment slightly decreased observed plant species diversity (P < 0.0001) (E) (Table S1). In contrast, there was no significant temporal trend in the positive effect of elevated CO2 on productivity (P = 0.77) (B), partly because elevated CO2 did not significantly influence observed plant species diversity (P = 0.49) (E) (Table S1). Effects in B, E, and F were estimated by linear mixed effects models (Table S1); effects in C and D were estimated by a structural equation model. Differences in biomass (B) or number of grassland plant species (E) were quantified between enriched and ambient plots, with positive biomass differences indicating that enriched plots were more productive, and with negative species differences indicating that enriched plots had fewer species, than ambient plots. Biomass differences for diversity effects (F) were quantified between plots planted with 16 and 4 grassland plant species, with positive values indicating that diverse plots were most productive.
We found that the net effect of N enrichment on productivity diminished over time in part because it decreased grassland plant diversity (Fig. 2). In contrast, elevated CO2 did not cause a decrease in plant diversity and consistently promoted productivity over time. N enrichment and elevated CO2 had similar direct effects (Fig. 2C), but different indirect effects (Fig. 2D), on productivity. The direct effects of N and CO2 enrichment on productivity were of comparable magnitude (F1,24 = 2.65, P = 0.117) and marginally significantly declined (Year: F1,24 = 4.01, P = 0.0567) to the same extent over time (Resource × Year: F1,24 = 0.06, P = 0.804) (Fig. 2C). In contrast, the indirect effects of N and CO2 enrichment significantly differed in magnitude (F1,24 = 113.58, P = 1.40 × 10−10) and significantly diverged over time (Year: F1,24 = 6.30, P = 0.0192; Resource × Year: F1,24 = 41.30, P = 1.21 × 10−6) (Fig. 2D). The indirect effects of N enrichment and elevated CO2 differed because N enrichment slightly decreased plant diversity, whereas elevated CO2 did not (Fig. 2E and Table S1). Species losses from N addition were nonrandom: there were fewer C4 grass, legume, and forb species, and slightly more C3 grass species, in enriched than in ambient N plots (5). The indirect effect of N enrichment became increasingly negative over time (Fig. 2D). This occurred because the positive effect of planted diversity on productivity became larger over time (Fig. 2F and Table S1) (13), whereas the effect of N enrichment on the observed number of species remained consistently negative (Fig. 2E and Table S1). The indirect effect of elevated CO2 became somewhat positive during later years because elevated CO2 slightly, although nonsignificantly, promoted diversity (Fig. 2E and Table S1), and diversity promoted productivity (Fig. 2F and Table S1) during later years. Thus, in contrast with N enrichment, elevated CO2 seems to influence productivity more by altering physiological responses than by altering the numbers or relative abundances of plant species (25).
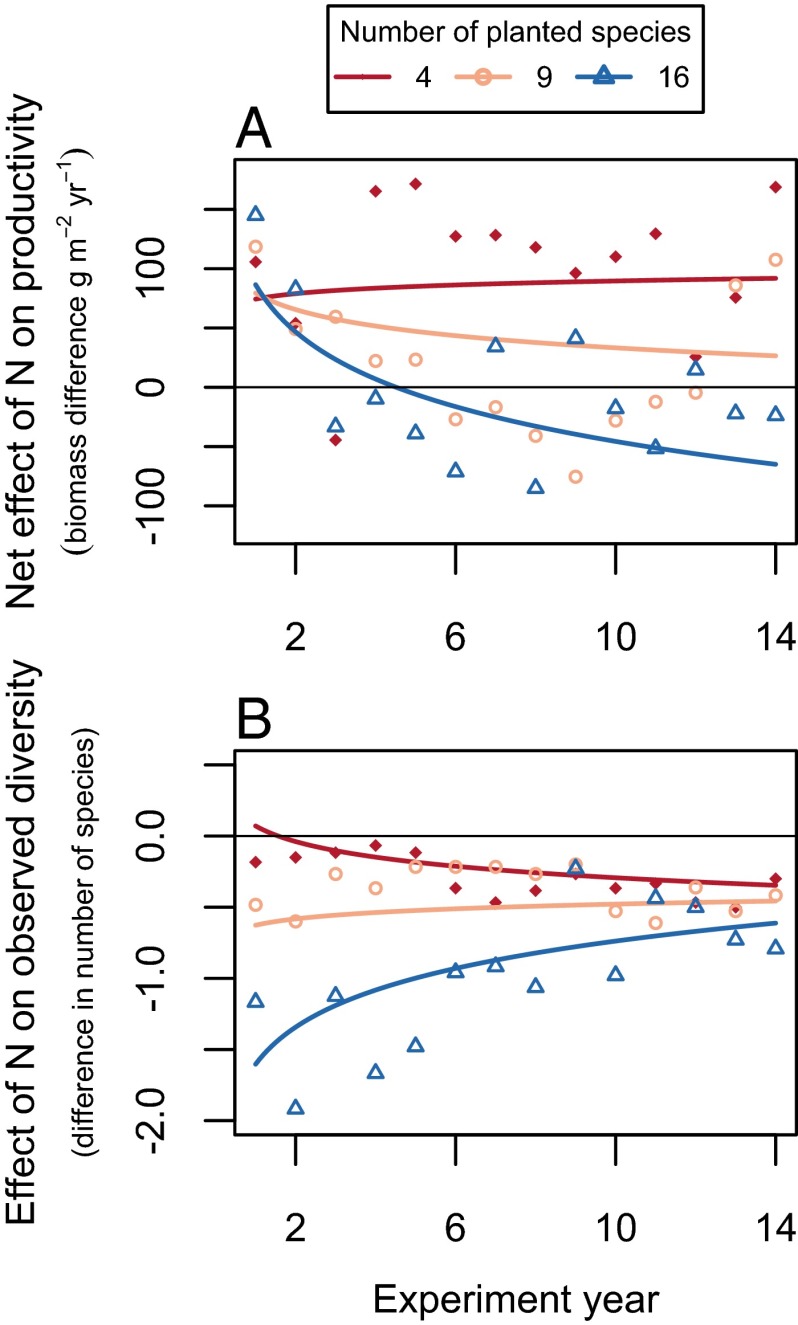
A comparison of BioCON plots planted with different numbers of species shows that the indirect effects of global changes, as mediated by changes in biodiversity, are of greater importance in more diverse communities (Fig. 3). Specifically, plots planted with the most species exhibited the largest declines in the net effect of N enrichment on productivity (Fig. 3A and Table S1), the largest negative effects of N enrichment on observed diversity (Fig. 3B and Table S1), and the largest positive effects of diversity on productivity (Table S1) (13, 15). In contrast, plots planted with only four species had the smallest decrease in diversity from N addition (Fig. 3B) and had no detectable decline through time in the effect of N enrichment on productivity (Fig. 3A).
Fig. 3.
(A and B) BioCON plots planted with the most grassland plant species exhibited the largest declines in the net effect of N enrichment on productivity (N × Year × Diversity: F1, 2056 = 20.52, P < 0.0001; Table S1) (A), and the largest negative effects of N enrichment on observed diversity (N × Year × Diversity: F1, 2056 = 7.79, P = 0.0053; Table S1) (B). Linear mixed effects models were used to estimate effects (Table S1). Differences in biomass (A) or number of species (B) were quantified between enriched and ambient plots, with positive biomass differences indicating that enriched plots were more productive, and with negative species differences indicating that enriched plots had fewer species, than ambient plots.
Chronic N enrichment influences productivity in many ways that are independent of its effects on diversity, and some of these effects may be confounded with its indirect effects via biodiversity. For example, N enrichment may simultaneously decrease biodiversity, soil pH, and legume biomass. All three of these effects could lead to diminishing positive net effects of N enrichment on productivity. To account for these potentially confounded effects, we refit our mixed effects model (Table S1) with soil pH and legume biomass included as covariates and tested whether there were still significant N × Year interactions. We found that the positive net effect of N enrichment on productivity still significantly diminished over time (N × Year: F1,1215 = 6.96, P = 0.0084; N × Diversity × Year: F1,1215 = 6.41, P = 0.0115), after accounting for the effects of soil pH (F1,1215 = 3.21, P = 0.0735) and legume biomass (F1,1215 = 616.41, P < 0.0001). Thus, our results are consistent with previous findings that fertilization can have diminishing returns for forage yield (33) due to soil acidification and shifts in community composition (e.g., reduced legume abundance) (34) and additionally show that these diminishing returns are also partly caused by negative effects of fertilization on biodiversity and positive effects of biodiversity on productivity.
Results of these two long-term nutrient enrichment experiments were strikingly similar, despite many differences between their designs. The N addition rate in the Nitrogen Enrichment Experiment that most closely corresponds to that in the BioCON experiment showed a comparable decrease in the positive net effect of N enrichment on productivity (compare lightest blue line in Fig. 1A with red line in Fig. 2B). N enrichment can result in both species losses and species colonizations (7). Our results for the BioCON experiment address the former, but not the latter, because potential colonists were removed as part of the study design. If the productivity of colonizing species were to compensate for that of lost species, then nutrient enrichment might continue to promote productivity in natural grasslands, even if it results in species losses. However, we found net losses of species and associated declines in productivity over time when our grasslands experienced nutrient enrichment (Fig. 1 C and D).
Our results offer an explanation for diminishing returns of plant productivity under chronic nutrient enrichment—a phenomenon that has been studied for more than a century (33). It has been long known that chronic fertilization can lead to diminishing yield returns due to soil acidification (34). Here we found evidence that such diminishing returns could also be caused by nonrandom species losses. Although our natural grassland plots were limed and received base cations, there were diminishing returns of productivity (Fig. 1A) that were associated with species losses (Fig. 1 B and D). Therefore, combining strategies for preventing soil acidification (e.g., repeated liming) with strategies for biodiversity conservation or restoration (e.g., repeated seed additions) may further sustain the positive effects of fertilization on forage yield in diverse pastures and hay meadows. Alternatively, given that augmentation of plant diversity could sustainably increase grassland productivity at least as much as fertilization (15), it may provide an environmentally beneficial alternative to fertilization. This is not to say that conserving the most diverse places will necessarily conserve the most productive places (e.g., productivity was greatest in plots with the fewest species in 2008; Fig. 1C) (34) but instead that maintaining or augmenting diversity over time within a managed ecosystem might also maintain or augment productivity over time in that ecosystem (e.g., productivity decreased least in plots that lost the fewest species between 1982 and 2008; Fig. 1D). Many ecosystems have already experienced chronic nutrient enrichment for decades (2, 35), and this has already decreased plant diversity (26, 36, 37). Thus, our results also suggest that chronic N deposition and fertilization promote productivity less than was previously thought in grasslands and perhaps other ecosystem types because of the loss of biodiversity associated with nutrient enrichment.
Our results also help resolve seemingly conflicting results from previous studies that have considered how biodiversity and productivity should covary across environmental gradients. Nutrient addition studies have been interpreted as showing that higher productivity (1, 6) is associated with decreased biodiversity (5, 26, 36, 37). In contrast, biodiversity experiments studies have been interpreted as showing that decreased plant diversity leads to lower productivity (8–15). Furthermore, a recent observational study found no association between productivity and plant diversity (38). These seemingly conflicting results can be reconciled by considering how diversity and productivity covary across both time and space. During the first year of our experiment in natural grasslands, nutrient enrichment promoted productivity (Fig. 1A) and decreased plant diversity (Fig. 1B), leading to a negative covariance over time between diversity and productivity shortly after the onset of nutrient enrichment that is consistent with results from many nutrient addition studies (6, 7, 33). After year 1, productivity was diminished (Fig. 1A) as plant species were lost (Fig. 1B), leading to a positive covariance over time between diversity and productivity (Fig. 1D) that is consistent with results from many biodiversity experiments (5, 26, 36, 37). In contrast, many previous observational studies (38) have focused on how diversity and productivity covary across space within time. Within the later years of our experiment, diversity and productivity negatively covaried across our fertility gradient (compare across tips of arrows in Fig. 1C). Interestingly, however, this relationship seems to be flattening out over time and may eventually lead to no association between diversity and productivity across plots, which would be consistent with results from a recent observational study (38). Therefore, results from these previous studies are not necessarily conflicting and may instead provide complementary insights that contribute to a comprehensive understanding of how diversity and productivity covary across time and space.
Our results show that changes in biodiversity can be an important intermediary driver of the long-term effects of human-caused environmental changes on ecosystem functioning. Anthropogenic N deposition and atmospheric CO2 concentrations have increased globally in recent decades and will likely further increase in the coming decades (1, 2, 35). In addition to resource enrichment, human activities are impacting ecosystems via land use changes, changes in temperature and precipitation, exotic species invasions, changes in disturbance frequency and intensity, and losses of native predators (1, 9, 15, 39). Our results suggest that knowing the effects of these global environmental changes on biodiversity can be essential to understanding their long-term impacts on ecosystem functioning and services. New policies and conservation strategies are needed to minimize the extent to which global environmental changes will erode the provision of ecosystem services by driving biodiversity declines.
Methods
Experimental Designs.
The long-term N addition experiments (“E001” and “E002” at Cedar Creek Ecosystem Science Reserve, Minnesota, United States, www.cbs.umn.edu/cedarcreek/research/experiments) were conducted in three successional grasslands and in a native savannah grassland (7), all within 5 km of one another and of the Cedar Creek Biodiversity and BioCON experiments (see below). As in previous studies (26), here we only included the two later successional prairie-like grassland fields (fields B and C, abandoned from agriculture in 1957 and 1934, respectively) and the prairie opening in native savannah (field D, never cultivated) in the analyses because the youngest field (field A, abandoned from agriculture in 1968) was relatively species poor and dominated by exotic invasive grasses at the beginning of the experiment. Each of the successional grasslands included a set of study plots on both existing and disturbed (disked) vegetation (7). Plots received annual wet N deposition of ∼6 kg ha−1 y−1 (58% NH4+, 42% NO3−) and fertilizer N (pelletized NH4NO3) at rates of 0, 10, 20, 34, 54, 95, 170, or 270 kg N ha−1 y−1 from 1982 to 2008 (26). To ensure primary limitation by N availability, plots also received P, K, Ca, Mg, and trace metals, none of which are limiting (7). In addition to the control plots that received these other nutrients but no N, there were also unamended plots that received no nutrients. For each of the nine treatments, there were six replicates in each successional grassland site (i.e., disked or undisked fields B and C; 9 treatments × 6 replicates × 4 fields = 216 prairie grassland plots) and five replicates in the undisked native savannah grassland (9 treatments × 5 replicates = 45 savannah grassland plots). All plots were 4 m by 4 m. Crushed limestone was added to plots as necessary to maintain constant soil pH. We considered only plots that were continuously fertilized throughout the study. That is, we excluded observations of plots after N treatments were ceased (half of the plots in disked field C after 1991). We also excluded observations with more than 2,000 g m−2 of biomass (nine cases) because these outliers included biomass of woody species that would not be a proxy for net annual aboveground primary productivity. We measured the number of plant species and biomass in each plot every year from 1982 to 1994, and at least 2 of every 3 years from 1995 to 2004, and in 2008. See previous publications for additional information (7, 26).
The Cedar Creek Biodiversity experiment (“E120” at Cedar Creek Ecosystem Science Reserve, Minnesota, United States, see Web address above) was established in 1994 by planting field plots with different numbers and combinations of perennial grassland species (14, 28). We randomly assigned plots to be seeded with 1, 2, 4, 8, or 16 perennial grassland species, with 30, 28, 29, 30, and 35 replicates, respectively, of the diversity levels. As in previous studies (13, 15), here we consider the 152 field plots (each 9 m by 9 m) receiving uniform fire treatment and without oak species. The 16 perennial grassland study species included four C4 grasses, four C3 grasses, four N-fixing legumes, and four nonleguminous forb species. During 2008, aboveground biomass was harvested by clipping four 0.1 m by 6.0 m strips per plot just above the soil surface. These peak biomass samples approximate aboveground annual net primary productivity (all aboveground biomass dies during winter). See previous publications for additional information (14, 28).
The BioCON experiment (“E141” at Cedar Creek Ecosystem Science Reserve, Minnesota, United States, see Web address above) was established by planting 296 field plots (each 2 m by 2 m) containing different numbers and combinations of perennial grassland species under ambient and elevated atmospheric CO2 and with either ambient or enriched soil N supply (5, 6, 13, 29). Plots were arranged in six circular 20-m-diameter rings. In three elevated-CO2 rings, a free-air CO2 enrichment system was used during each growing season to elevate the CO2 concentration by ∼180 μmol mol−1 to concentrations likely to be reached later this century. Three ambient CO2 rings were treated identically but without additional CO2. Half of the plots in each ring received N amendments of 40 kg N ha−1 y−1 applied as NH4NO3 on three dates each year. The treatments were arranged in complete factorial combination of two levels of atmospheric CO2 (ambient and elevated), four levels of plant species diversity (1, 4, 9, and 16 species), and two levels of N (ambient and enriched). There were 32, 15, 15, and 12 plots with 1, 4, 9 and 16 species, respectively, at each of the four contrasting CO2 and N levels (n = 296 plots, including 128 monocultures and 168 mixtures). Here we analyzed N and CO2 treatment effects in the species mixture plots (n = 168), where realized diversity changed during the study. We excluded 6 of the 15 plots planted with nine species during years 2007–2011, while they were part of a water manipulation study. The 16 study species included four C4 grasses, four C3 grasses, four N-fixing legumes, and four nonleguminous forb species. Each year in every plot aboveground biomass was harvested by clipping a 10 cm by 100 cm strip just above the soil surface in June and August. Here we present only the August data, because these peak biomass samples approximate aboveground annual net primary productivity (all aboveground biomass dies during winter). The observed number of species (i.e., species richness) was the mean of values observed in peak biomass samples and percent cover estimates. See previous publications for additional information (5, 6, 13, 29). Our study both (i) experimentally manipulated biodiversity and global environmental change factors, which is necessary to establish the three causal pathways shown in Fig. 2A, and (ii) teased apart the net effects of global environmental change factors into direct and indirect components. Some previous studies have done either the first (40) or the second (41–43) of these steps.
Statistical Analyses.
All statistical analyses were conducted in R 2.15.1 (44). We first considered temporal trends in the effects of N fertilization on diversity and productivity in natural grasslands. This allowed us to test whether the net effect of fertilization on productivity decreased over time when other species could colonize and when soils were limed. We conducted an analysis of covariance to test whether differences in biomass or numbers of species across years depended on year, N addition rate, or their interaction (Fig. 1).
We used structural equation modeling to quantify the relative magnitudes of direct and indirect effects. Analyses of structural equation models can range from exploratory analyses, whereby the initial hypothesized model is loosely based on theory and is modified to improve the fit between model and data, to confirmatory analyses, whereby a single model that is based on prior theoretical knowledge is tested with data (45). We conducted a confirmatory analysis of a single structural equation model that was based on previous results from theoretical and experimental studies (41). We fit the model shown in Fig. S1 within each year to the mixture data from the BioCON experiment. N and CO2 enrichment treatments were coded as a binary variable that indicated the ambient (0) or enriched (1) treatment level. These indicator values were uncorrelated because treatments were randomly assigned to experimental units. Productivity and observed diversity were ln-transformed to meet linear model assumptions. The model was fit with the sem function, and standardized regression coefficients were extracted with the std.coef function, from the sem package of R. We used a χ2 test to determine whether there was a significant deviation between the observed covariance matrix and that predicted by the model. In all years, we found no significant deviation between the observed and expected covariance matrix (in all years: χ2 < 0.001, P > 0.99), indicating that the structural equation model could not be rejected as a potential explanation of the observed data. We then used analysis of covariance to test whether the standardized effect sizes depended on Year, Resource (N or CO2), or their interaction.
Our confirmatory structural equation model was useful for quantifying the relative magnitudes of the direct and indirect effects. However, we cannot infer from these results that changing diversity caused a change in productivity, rather than vice versa, because we simply assumed causal pathways and considered the correlations between the observed diversity and productivity values across mixture plots. Previous studies have assumed similar (41) or different (42) causal pathways between nutrient availability, productivity, and diversity; however, none of these previous studies have experimentally tested all of the hypothesized causal pathways. The BioCON experiment (5, 6, 13, 29) fully crossed species diversity (1, 4, 9, or 16 species), N (enriched or ambient), and CO2 (elevated or ambient) treatments. This allowed us to test the effect of changing diversity on productivity and the effect of resource enrichment on productivity and observed diversity.
We fit a linear mixed effects model to test for temporal trends in the effects of BioCON treatments (N, CO2, and Diversity) on response variables (productivity and observed number of species). This analysis accounted for the split-plot treatment design and the repeated measurements. The CO2 main effect was tested against the variation across rings (main plot); the N main effect, Diversity main effect, and all interactions between the CO2, N, and Diversity treatments were tested against the variation across plots within rings (split-plot); and the Year main effect and all interactions with Year were tested against the variation across all observations within plots within rings. Table S1 shows the corresponding degrees of freedom. We assumed a compound symmetry correlation structure because this structure resulted in the best fit according to Akaike’s information criterion. The model was fit using the lme function in the nlme package in R.
Supplementary Material
Acknowledgments
We thank Troy Mielke, Dan Bahauddin, Kally Worm, and many summer interns for their assistance with this research, and reviewers for insightful comments on the manuscript. This work was supported by Grant DE-FG02-96ER62291 from the US Department of Energy Program for Ecosystem Research, Grant DE-FC02-06ER64158 from the US Department of Energy National Institute for Climatic Change Research, Grants DEB-9411972, DEB-0080382, and DEB-0620652 from the US National Science Foundation Long-Term Ecological Research Program, Grant DEB-0322057 from the US National Science Foundation Biocomplexity Coupled Biogeochemical Cycles Program, Grant DEB-0716587 from the US National Science Foundation Long-Term Research in Environmental Biology Program, and by the University of Minnesota.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data sets used in this study are available at the Cedar Creek Ecosystem Science Reserve Web site, www.cbs.umn.edu/cedarcreek/research/data.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310880110/-/DCSupplemental.
References
- 1.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277(5325):494–499. [Google Scholar]
- 2.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 3.Vitousek PM, et al. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl. 1997;7(3):737–750. [Google Scholar]
- 4.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451(7176):293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 5.Reich PB. Elevated CO2 reduces losses of plant diversity caused by nitrogen deposition. Science. 2009;326(5958):1399–1402. doi: 10.1126/science.1178820. [DOI] [PubMed] [Google Scholar]
- 6.Reich PB, Hobbie SE. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat Climate Change. 2013;3(3):278–282. [Google Scholar]
- 7.Tilman D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr. 1987;57(3):189–214. [Google Scholar]
- 8.Hector A, et al. Plant diversity and productivity experiments in european grasslands. Science. 1999;286(5442):1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 9.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 10.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477(7363):199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 11.Loreau M. From Populations to Ecosystems: Theoretical Foundations for a New Ecological Synthesis. Princeton: Princeton Univ Press; 2010. [Google Scholar]
- 12.Naeem S, Bunker DE, Hector A, Loreau M, Perrings C. Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 13.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336(6081):589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 14.Tilman D, et al. Diversity and productivity in a long-term grassland experiment. Science. 2001;294(5543):843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 15.Tilman D, Reich PB, Isbell F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc Natl Acad Sci USA. 2012;109(26):10394–10397. doi: 10.1073/pnas.1208240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balvanera P, et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett. 2006;9(10):1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JE. Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ. 2009;7(8):437–444. [Google Scholar]
- 19.Srivastava DS, Vellend M. Biodiversity-ecosystem function research: Is it relevant to conservation? Annu Rev Ecol Evol Syst. 2005;36(1):267–294. [Google Scholar]
- 20.Wardle DA, Jonsson M. Biodiversity effects in real ecosystems—a response to Duffy. Front Ecol Environ. 2010;8(1):10–11. [Google Scholar]
- 21.Isbell FI, Losure DA, Yurkonis KA, Wilsey BJ. Diversity-productivity relationships in two ecologically realistic rarity and extinction scenarios. Oikos. 2008;117(7):996–1005. [Google Scholar]
- 22.Smith MD, Knapp AK. Dominant species maintain ecosystem function with non-random species loss. Ecol Lett. 2003;6(6):509–517. [Google Scholar]
- 23.Zavaleta ES, Hulvey KB. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science. 2004;306(5699):1175–1177. doi: 10.1126/science.1102643. [DOI] [PubMed] [Google Scholar]
- 24.Hillebrand H, Matthiessen B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol Lett. 2009;12(12):1405–1419. doi: 10.1111/j.1461-0248.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith MD, Knapp AK, Collins SL. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology. 2009;90(12):3279–3289. doi: 10.1890/08-1815.1. [DOI] [PubMed] [Google Scholar]
- 26.Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451(7179):712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- 27.Wedin DA, Tilman D. Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science. 1996;274(5293):1720–1723. doi: 10.1126/science.274.5293.1720. [DOI] [PubMed] [Google Scholar]
- 28.Tilman D, et al. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277(5330):1300–1302. [Google Scholar]
- 29.Reich PB, et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature. 2001;410(6830):809–812. doi: 10.1038/35071062. [DOI] [PubMed] [Google Scholar]
- 30.Collins SL, et al. Rank clocks and plant community dynamics. Ecology. 2008;89(12):3534–3541. doi: 10.1890/07-1646.1. [DOI] [PubMed] [Google Scholar]
- 31.Isbell F, Tilman D, Polasky S, Binder S, Hawthorne P. Low biodiversity state persists two decades after cessation of nutrient enrichment. Ecol Lett. 2013;16(4):454–460. doi: 10.1111/ele.12066. [DOI] [PubMed] [Google Scholar]
- 32.Loreau M. Biodiversity and ecosystem functioning: A mechanistic model. Proc Natl Acad Sci USA. 1998;95(10):5632–5636. doi: 10.1073/pnas.95.10.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawes JB, Gilbert JH. Agricultural, botanical, and chemical results of experiments on the mixed herbage of permanent meadow, conducted for more than twenty years in succession on the same land. Part I. Philos Trans R Soc Lond. 1880;171(1):289–416. [Google Scholar]
- 34.Silvertown J, et al. The Park Grass Experiment 1856-2006: Its contribution to ecology. J Ecol. 2006;94(4):801–814. [Google Scholar]
- 35.Galloway J, et al. Nitrogen cycles: Past, present, and future. Biogeochemistry. 2004;70(2):153–226. [Google Scholar]
- 36.Phoenix GK, et al. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob Change Biol. 2006;12(3):470–476. [Google Scholar]
- 37.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Impact of nitrogen deposition on the species richness of grasslands. Science. 2004;303(5665):1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- 38.Adler PB, et al. Productivity is a poor predictor of plant species richness. Science. 2011;333(6050):1750–1753. doi: 10.1126/science.1204498. [DOI] [PubMed] [Google Scholar]
- 39.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island; 2005. [Google Scholar]
- 40.Fridley JD. Resource availability dominates and alters the relationship between species diversity and ecosystem productivity in experimental plant communities. Oecologia. 2002;132(2):271–277. doi: 10.1007/s00442-002-0965-x. [DOI] [PubMed] [Google Scholar]
- 41.Cardinale BJ, Hillebrand H, Harpole WS, Gross K, Ptacnik R. Separating the influence of resource ‘availability’ from resource ‘imbalance’ on productivity-diversity relationships. Ecol Lett. 2009;12(6):475–487. doi: 10.1111/j.1461-0248.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 42.Grace JB, et al. Does species diversity limit productivity in natural grassland communities? Ecol Lett. 2007;10(8):680–689. doi: 10.1111/j.1461-0248.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 43.Laliberté E, Tylianakis JM. Cascading effects of long-term land-use changes on plant traits and ecosystem functioning. Ecology. 2012;93(1):145–155. doi: 10.1890/11-0338.1. [DOI] [PubMed] [Google Scholar]
- 44.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 45.Grace JB. Structural Equation Modeling and Natural Systems. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.