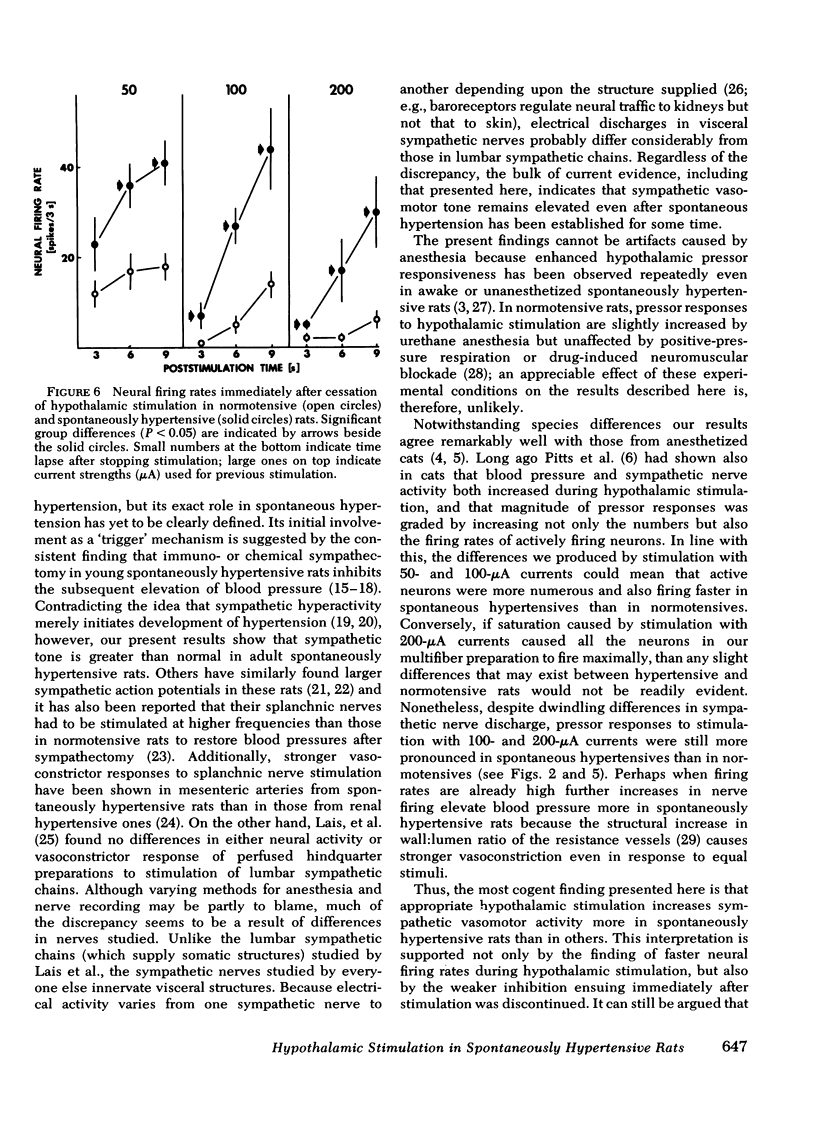
Abstract
To determine whether sympathetic hyperactivity of hypothalamic origin contributes to keep blood pressures high in spontaneous hypertension, aortic pressures and sympathetic nerve spike potentials were recorded during electrical stimulation of the posterior hypothalamus in urethane-anesthetized normotensive or hypertensive rats. Basal sympathetic nerve activity was higher in spontaneously hypertensive rats than in either normotensive or deoxycorticosterone acetate-salt hypertensive ones even before stimulation began. Blood pressure elevations produced by hypothalamic stimulation were always preceded by substantial increases in amplitude and rate of neural firing. Changes in amplitude could not be quantified, but rates of neural firing accelerated much more in spontaneous hypertensives than in normotensives during stimulation with 50- and 100-μA currents. Similar differences between deoxycorticosterone acetate-salt hypertensives and either normotensives or spontaneous hypertensives were not statistically significant. Nerve activity invariably became quiescent immediately after hypothalamic stimulation was discontinued, and recovery from this poststimulatory inhibition was faster in spontaneously hypertensive than in normotensive rats. Although spontaneous hypertensives generally also had stronger pressor responses to various sympathomimetic stimuli, responses to hypothalamic stimulation were enhanced to a greater extent than those to either norepinephrine or sympathetic nerve stimulation. Because this selectivity indicates participation of mechanisms other than augmented cardiovascular reactivity, further enhancement of responsiveness to hypothalamic stimuli was attributed to the associated increase in sympathetic nerve firing. These results are in accord with the hypothesis that the blood pressure elevation in rats with established spontaneous hypertension is a result, at least in part, of sympathetic hyperactivity emanating from the posterior hypothalamus.
Full text
PDF

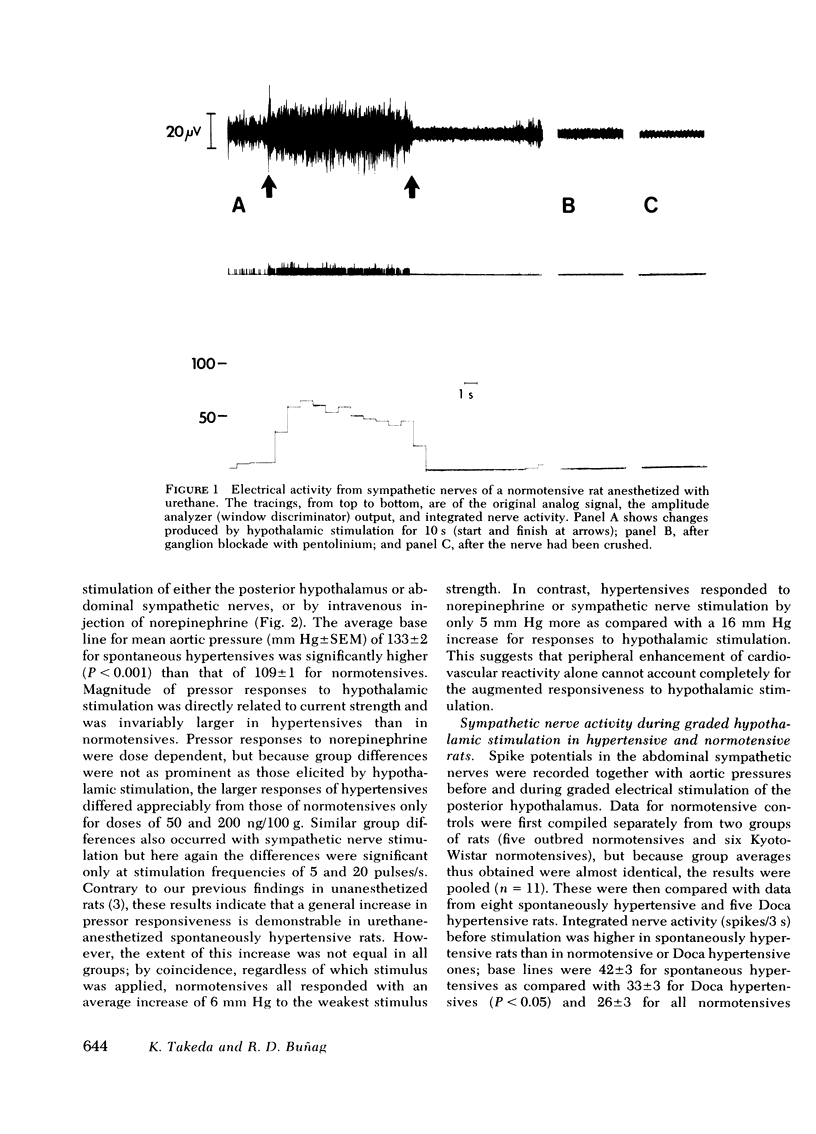
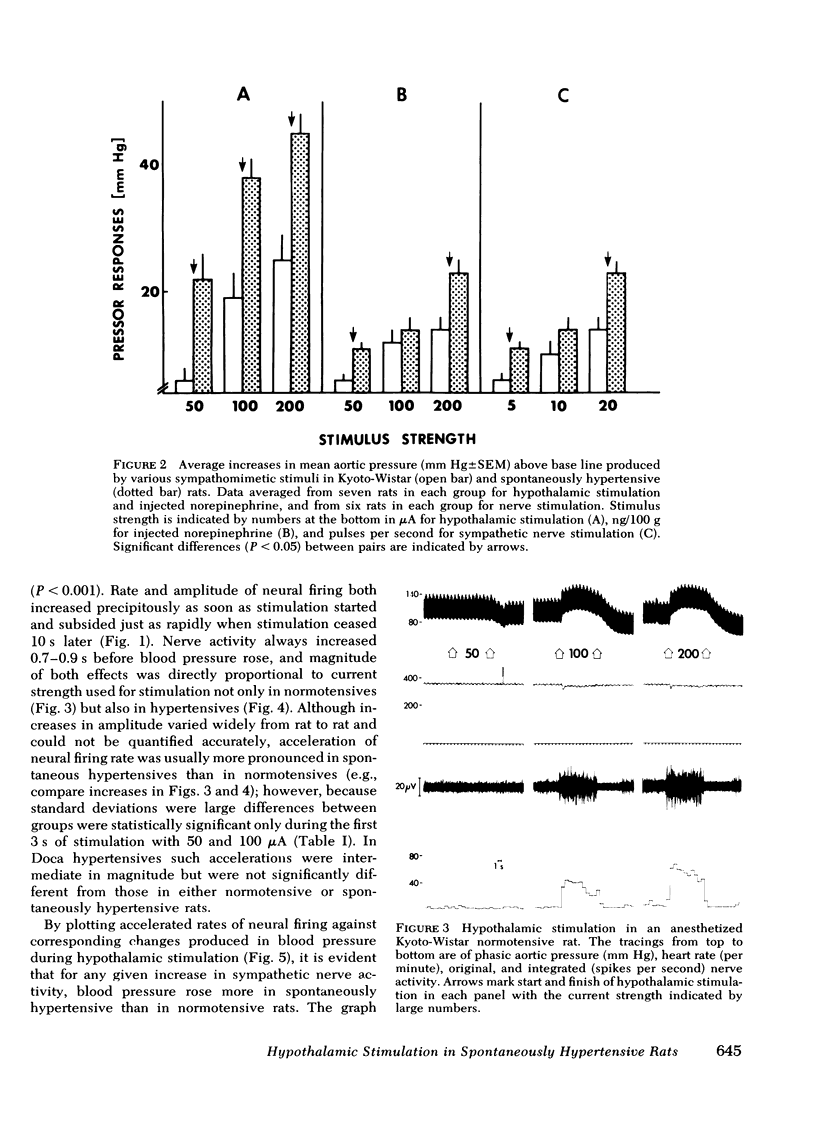
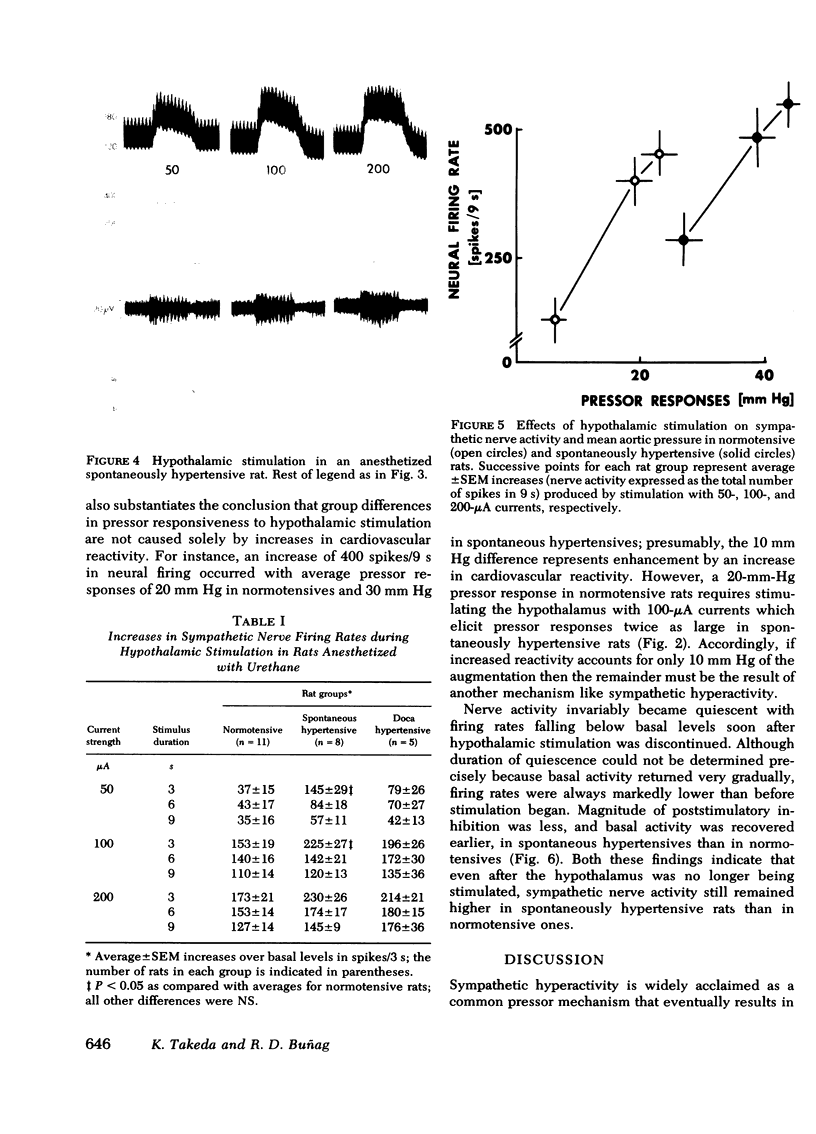

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunag R. D., Eferakeya A. E., Langdon D. S. Enhancement of hypothalamic pressor responses in spontaneously hypertensive rats. Am J Physiol. 1975 Jan;228(1):217–222. doi: 10.1152/ajplegacy.1975.228.1.217. [DOI] [PubMed] [Google Scholar]
- Buñag R. D., Eferakeya J. E. Reduced cardiovascular responsiveness to hypothalamic stimulation during urethane and amobarbital anesthesia. Pharmacology. 1973;10(3):143–151. doi: 10.1159/000136434. [DOI] [PubMed] [Google Scholar]
- Buñag R. D., Riley E., Montello M. Sustained pressore responsiveness to prolonged hypothalamic stimulation in awake rats. Am J Physiol. 1976 Dec;231(6):1708–1715. doi: 10.1152/ajplegacy.1976.231.6.1708. [DOI] [PubMed] [Google Scholar]
- Buñag R. D. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J Appl Physiol. 1973 Feb;34(2):279–282. doi: 10.1152/jappl.1973.34.2.279. [DOI] [PubMed] [Google Scholar]
- Eferakeya A., Buñag R. D. Adrenomedullary pressor responses during posterior hypothalamic stimulation. Am J Physiol. 1974 Jul;227(1):114–118. doi: 10.1152/ajplegacy.1974.227.1.114. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Weiss L. Background of increased flow resistance and vascular reactivity in spontaneously hypertensive rats. Acta Physiol Scand. 1970 Sep;80(1):93–106. doi: 10.1111/j.1748-1716.1970.tb04773.x. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Weiss L. The effects of "immunosympathectomy" on blood pressure and vascular "reactivity" in normal and spontaneously hypertensive rats. Acta Physiol Scand. 1972 Apr;84(4):512–523. doi: 10.1111/j.1748-1716.1972.tb05202.x. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Activation of the central pathway of the baroreceptor reflex, a possible mechanism of the hypotensive action of clonidine. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):231–246. doi: 10.1007/BF00500285. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Finch L., Thoenen H. Central adrenergic neurones and the initiation and development of experimental hypertension. Experientia. 1972 Oct 15;28(10):1200–1203. doi: 10.1007/BF01946170. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Haefely W. Pre- and postjunctional supersensitivity of the mesenteric artery preparation from normotensive and hypertensive rats. Naunyn Schmiedebergs Arch Pharmakol. 1970;266(1):18–33. doi: 10.1007/BF00997779. [DOI] [PubMed] [Google Scholar]
- Hallbäck M., Folkow B. Cardiovascular responses to acute mental 'stress' in spontaneously hypertensive rats. Acta Physiol Scand. 1974 Apr;90(4):684–698. doi: 10.1111/j.1748-1716.1974.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Iriuchijima J. Sympathetic discharge rate in spontaneously hypertensive rats. Jpn Heart J. 1973 Jul;14(4):350–356. doi: 10.1536/ihj.14.350. [DOI] [PubMed] [Google Scholar]
- Judy W. V., Watanabe A. M., Henry D. P., Besch H. R., Jr, Murphy W. R., Hockel G. M. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976 Jun;38(6 Suppl 2):21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- Lais L. T., Shaffer R. A., Brody M. J. Neurogenic and humoral factors controlling vascular resistance in the spontaneously hypertensive rat. Circ Res. 1974 Nov;35(5):764–774. doi: 10.1161/01.res.35.5.764. [DOI] [PubMed] [Google Scholar]
- Nathan M. A., Reis D. J. Hypoxemia, atelectasis, and the elevation of arterial pressure and heart rate in paralyzed artificially ventilated rat. Life Sci. 1975 Apr 1;16(7):1103–1120. doi: 10.1016/0024-3205(75)90195-2. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Irisawa A., Nisimaru N. Nonuniformity of sympathetic nerve activity to the skin and kidney. Am J Physiol. 1973 Feb;224(2):256–264. doi: 10.1152/ajplegacy.1973.224.2.256. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Judy W. V., Wilson M. F. Hypothalamic stimulus effects on sympathetic nerve activity. Am J Physiol. 1970 Feb;218(2):453–462. doi: 10.1152/ajplegacy.1970.218.2.453. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963 Mar;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nosaka S., Yamori Y., Matsumoto M. Participation of neural factor in the pathogenesis of hypertension in the spontaneously hypertensive rat. Jpn Heart J. 1967 Mar;8(2):168–180. doi: 10.1536/ihj.8.168. [DOI] [PubMed] [Google Scholar]
- Vapaatalo H., Hackman R., Anttila P., Vainionpä V., Neuvonen P. J. Effects of 6-hydroxydopamine on spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 1974;284(1):1–13. doi: 10.1007/BF00499968. [DOI] [PubMed] [Google Scholar]
- Yamori Y., Matsumoto M., Yamabe H., Okamoto K. Augmentation of spontaneous hypertension by chronic stress in rats. Jpn Circ J. 1969 Apr;33(4):399–409. doi: 10.1253/jcj.33.399. [DOI] [PubMed] [Google Scholar]
- Yamori Y., Okamoto K. Hypothalamic tonic regulation of blood pressure in spontaneously hypertensive rats. Jpn Circ J. 1969 May;33(5):509–519. doi: 10.1253/jcj.33.509. [DOI] [PubMed] [Google Scholar]