Abstract
Natural measles causes prolonged depression of cell-mediated immunity yet little is known as to how the infection influences lymphocyte function. Therefore, we studied the properties and function of lymphocytes during and after measles. The number and proportion of circulating thymus-derived lymphocytes was low during the acute stage of measles, and at this time 37% of these cells showed positive immunofluorescent staining for measles virus after stimulation with phytohemagglutinin. 7% of B cells were shown to contain virus but their numbers did not alter during the infection. Acute-phase lymphocytes, when stimulated, yielded infective virus and half were killed on incubation with autologous serum and complement. In acute measles the increase in [3H]-thymidine uptake of lymphocytes when stimulated with an optimal dose of PHA was normal in media with 10% fetal calf serum and low in media containing 10% autologous serum: the mean values were 56.8±34.1 and 23.7±25.9 cpm × 103 per 106 lymphocytes, respectively. Stimulation of acute-phase lymphocytes by Candida antigen was also low in media containing autologous serum averaging 1.2 × 103 cpm per 106 lymphocytes. On recovery 4-6 wk later this rose significantly to 18.9±19.8. The mean migration index of leukocytes to heat-killed candida cells in acute measles was 0.84±SD 0.08, and this fell significantly to 0.75±SD 0.08 4 wk later. Thus, depletion of T cells, an inhibitor of lymphocyte proliferation in the serum and a possible defect in antigen processing, interacts to depress cell-mediated immunity in measles.
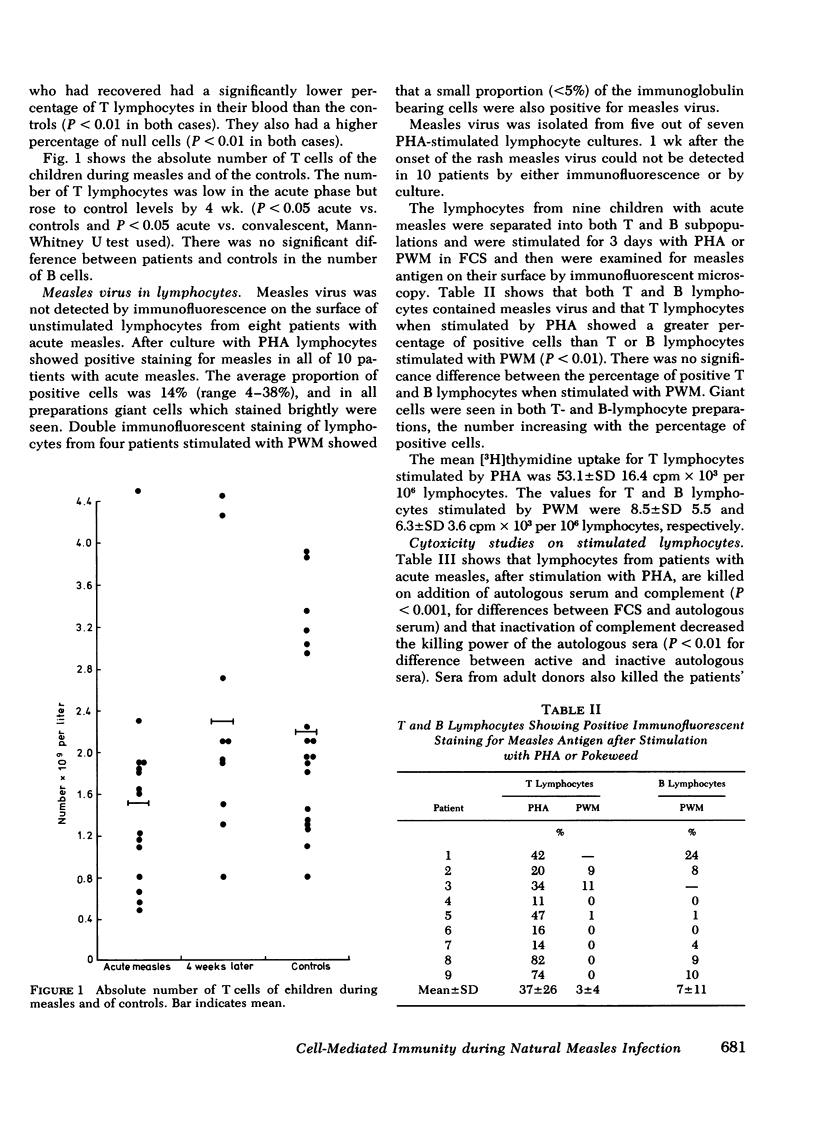
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Rabson A. R., Sher R., Koornhof H. J. Defective neutrophil motility in children with measles. J Pediatr. 1976 Jul;89(1):27–32. doi: 10.1016/s0022-3476(76)80921-3. [DOI] [PubMed] [Google Scholar]
- Dossetor J., Whittle H. C., Greenwood B. M. Persistent measles infection in malnourished children. Br Med J. 1977 Jun 25;1(6077):1633–1635. doi: 10.1136/bmj.1.6077.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Roffe L. M., Magrath I. T., Dumonde D. C. Standardization of the leucocyte migration test. Int Arch Allergy Appl Immunol. 1973;45(1):308–321. doi: 10.1159/000231048. [DOI] [PubMed] [Google Scholar]
- Morley D. Severe measles in the tropics. I. Br Med J. 1969 Feb 1;1(5639):297–contd. doi: 10.1136/bmj.1.5639.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newble D. I., Holmes K. T., Wangel A. G., Forbes I. J. Immune reactions in acute viral hepatitis. Clin Exp Immunol. 1975 Apr;20(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Cooke A. R., Ayeni O., Adejumo T. A. Studies on leukocyte cultures in measles. I. Lymphocyte transformation and giant cell formation in leukocyte cultures from clinical cases of measles. Arch Gesamte Virusforsch. 1974;44(4):313–322. [PubMed] [Google Scholar]
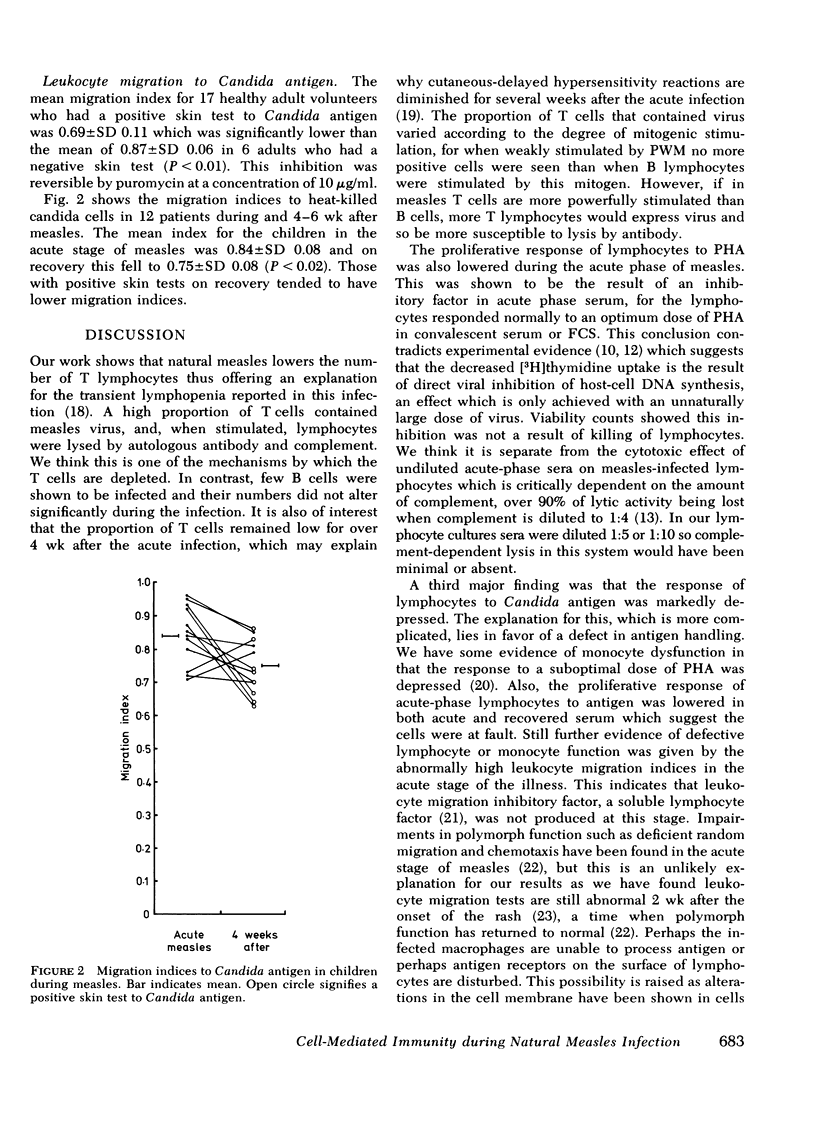
- Poste G., Reeve P. Increased mobility and redistribution of concanavalin A receptors on cells infected with Newcastle disease virus. Nature. 1974 Feb 15;247(5441):469–471. doi: 10.1038/247469a0. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Products of activated lymphocytes: leukocyte inhibitory factor (LIF) distinct from migration inhibitory factor (MIF). J Immunol. 1974 Apr;112(4):1461–1466. [PubMed] [Google Scholar]
- STARR S., BERKOVICH S. EFFECTS OF MEASLES, GAMMA-GLOBULIN-MODIFIED MEASLES AND VACCINE MEASLES ON THE TUBERCULIN TEST. N Engl J Med. 1964 Feb 20;270:386–391. doi: 10.1056/NEJM196402202700802. [DOI] [PubMed] [Google Scholar]
- Smithwick E. M., Berkovich S. In vitro suppression of the lymphocyte response to tuberculin by live measles virus. Proc Soc Exp Biol Med. 1966 Oct;123(1):276–278. doi: 10.3181/00379727-123-31465. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Albrecht P., Lucas S. J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975 May;114(5):1458–1461. [PubMed] [Google Scholar]
- Whittle H. C., Bradley-Moore A., Fleming A., Greenwood B. M. Effects of measles on the immune response of Nigerian children. Arch Dis Child. 1973 Oct;48(10):753–756. doi: 10.1136/adc.48.10.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweiman B. Effect of viable and non-viable measles virus on proliferating human lymphocytes. Int Arch Allergy Appl Immunol. 1972;43(4):600–607. doi: 10.1159/000230872. [DOI] [PubMed] [Google Scholar]
- Zweiman B. In vitro effects of measles virus on proliferating human lymphocytes. J Immunol. 1971 May;106(5):1154–1158. [PubMed] [Google Scholar]
- Zweiman B., Miller M. F. Effects of non-viable measles virus on proliferating human lymphocytes. 2. Characteristics of the suppressive reaction. Int Arch Allergy Appl Immunol. 1974;46(6):822–833. doi: 10.1159/000231184. [DOI] [PubMed] [Google Scholar]


