Abstract
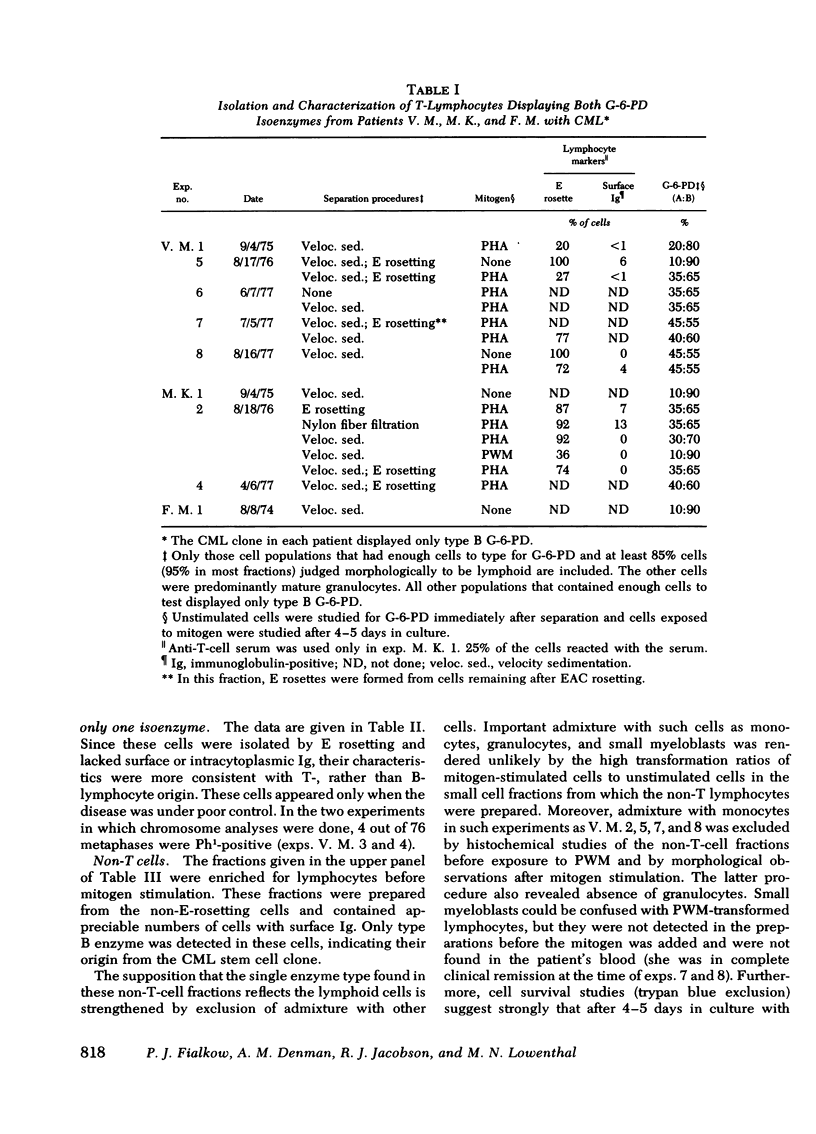
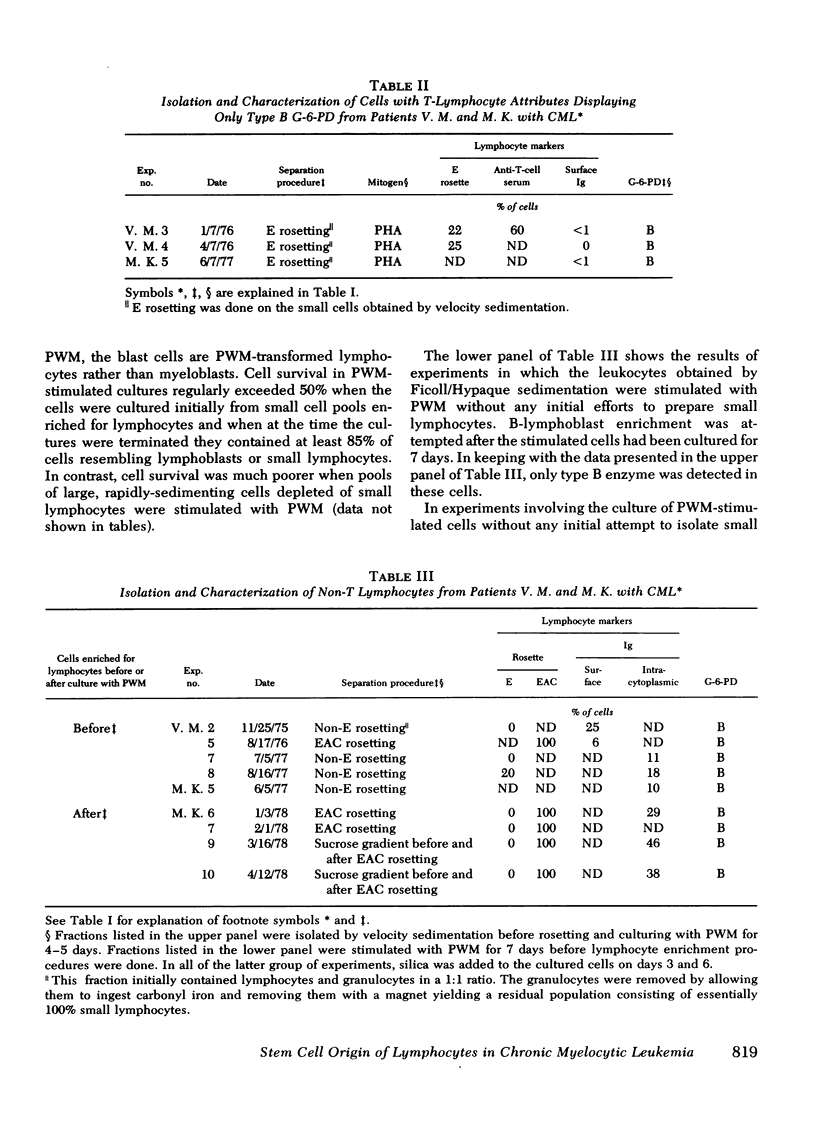
In three patients with chronic myelocytic leukemia who were heterozygous at the X-linked glucose-6-phospháte dehydrogenase locus, lymphocytes were studied to determine if they had the same stem cell origin as the leukemic myeloid cells. Normal tissues such as skin had both B and A glucose-6-phosphate dehydrogenase isoenzymes, but the leukemic myelogenous cells displayed only one isoenzyme type, consistent with their clonal origin. A population of cells with undoubted thymus-derived (T)-lymphocyte characteristics had both isoenzymes. Presumably, then, these T cells did not arise from the leukemic stem cell, either because they antedated the development of leukemia in that stem cell or, more likely, because they arose from progenitors not involved by the disease. In contrast, another population of lymphocytes showed only one isoenzyme type, suggesting that it arose from the chronic myelocytic leukemia stem cell. However, although this population contained many cells with the characteristics of bone marrow-derived (B) lymphocytes, it is not certain that the single enzyme produced by the cells over all can be attributed to B lymphocytes rather than to contaminating non-B-lymphoid cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEUTLER E., YEH M., FAIRBANKS V. F. The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci U S A. 1962 Jan 15;48:9–16. doi: 10.1073/pnas.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Skutelsky E., Kunkel H. G. Sheep red cell binding to human lymphocytes treated with neuraminidase; enhancement of T cell binding and identification of a subpopulation of B cells. J Exp Med. 1973 Jun 1;137(6):1532–1537. doi: 10.1084/jem.137.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing R., Rafizadeh B., Drew I., Hartman G., Gale R., Terasaki P. Human B-lymphocyte antigens expressed by lymphocytic and myelocytic leukemia cells. I. Detection by rabbit antisera. J Exp Med. 1976 Jul 1;144(1):167–178. doi: 10.1084/jem.144.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R. Editorial: Hematopoietic stem cell theory in relation to possible lymphoblastic conversion of chronic myeloid leukemia. Blood. 1974 Sep;44(3):449–453. [PubMed] [Google Scholar]
- Broom B. C., De la Concha E. G., Webster A. D., Janossy G. J., Asherson G. L. Intracellular immunoglobulin production in vitro by lymphocytes from patients with hypogammaglobulinaemia and their effect on normal lymphocytes. Clin Exp Immunol. 1976 Jan;23(1):73–77. [PMC free article] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Billing R. Antigens expressed by human B lymphocytes and myeloid stem cells. J Exp Med. 1977 Oct 1;146(4):1143–1145. doi: 10.1084/jem.146.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON R. G., NITOWSKY H. M., CHILDS B. DEMONSTRATION OF TWO POPULATIONS OF CELLS IN THE HUMAN FEMALE HETEROZYGOUS FOR GLUCOSE-6-PHOSPHATE DEHYDROGENASE VARIANTS. Proc Natl Acad Sci U S A. 1963 Sep;50:481–485. doi: 10.1073/pnas.50.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman A. M., Pelton B. K. Control mechanisms in infectious mononucleosis. Clin Exp Immunol. 1974 Sep;18(1):13–25. [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Miller R. G., Phillips R. A. Differentiation of rosette-forming cells from myeloid stem cells. J Immunol. 1970 Sep;105(3):719–729. [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gartler S. M., Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Lisker R., Detter J., Giblett E. R., Zavala C. 6-phosphogluconate dehydrogenase: hemizygous manifestation in a patient with leukemia. Science. 1969 Jan 10;163(3863):194–195. doi: 10.1126/science.163.3863.194. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet. 1973 Jul;37(1):39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Forman E. N., Padre-Mendoza T., Smith P. S., Barker B. E., Farnes P. Ph1-positive childhood leukemias: spectrum of lymphoid-myeloid expressions. Blood. 1977 Apr;49(4):549–558. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Roberts M., Greaves M. F. Target cell in chronic myeloid leukaemia and its relationship to acute lymphoid leukaemia. Lancet. 1976 Nov 13;2(7994):1058–1061. doi: 10.1016/s0140-6736(76)90970-3. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Marks S. M., Baltimore D., McCaffrey R. Terminal transferase as a predictor of initial responsiveness to vincristine and prednisone in blastic chronic myelogenous leukemia: a co-operative study. N Engl J Med. 1978 Apr 13;298(15):812–814. doi: 10.1056/NEJM197804132981503. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Harrison T. A., Parkman R., Baltimore D. Terminal deoxynucleotidyl transferase activity in human leukemic cells and in normal human thymocytes. N Engl J Med. 1975 Apr 10;292(15):775–780. doi: 10.1056/NEJM197504102921504. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- O'Rourke E. J., Halstead S. B., Allison A. C., Platts-Mills T. A. Specific lethality of silica for human peripheral blood mononuclear phagocytes, in vitro. J Immunol Methods. 1978;19(2-3):137–151. doi: 10.1016/0022-1759(78)90174-6. [DOI] [PubMed] [Google Scholar]
- Pelton B. K., Imrie R. C., Denman A. M. Susceptibility of human lymphocyte populations to infection by herpes simplex virus. Immunology. 1977 May;32(5):803–810. [PMC free article] [PubMed] [Google Scholar]
- Peterson L. C., Bloomfield C. D., Brunning R. D. Blast crisis as an initial or terminal manifestation of chronic myeloid leukemia. A study of 28 patients. Am J Med. 1976 Feb;60(2):209–220. doi: 10.1016/0002-9343(76)90430-7. [DOI] [PubMed] [Google Scholar]
- Propp S., Lizzi F. A. Philadelphia chromosome in acute lymphocytic leukemia. Blood. 1970 Sep;36(3):353–360. [PubMed] [Google Scholar]
- Ross G. D., Jarowski C. I., Rabellino E. M., Winchester R. J. The sequential appearance of Ia-like antigens and two different complement receptors during the maturation of human neutrophils. J Exp Med. 1978 Mar 1;147(3):730–744. doi: 10.1084/jem.147.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin P. S., Anderson P. N., Gallo R. C. Terminal deoxynucleotidyl transferase activities in human blood leukocytes and lymphoblast cell lines: high levels in lymphoblast cell lines and in blast cells of some patients with chronic myelogenous leukemia in acute phase. Blood. 1976 Jan;47(1):11–20. [PubMed] [Google Scholar]
- Schmidt R., Dar H., Santorineou M., Sekine I. Letter: Ph-1 chromosome and loss and reappearance of the Y chromosome in acute lymphocytic leukaemia. Lancet. 1975 May 17;1(7916):1145–1145. doi: 10.1016/s0140-6736(75)92536-2. [DOI] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- TOUGH I. M., JACOBS P. A., COURT BROWN W. M., BAIKIE A. G., WILLIAMSON E. R. Cytogenetic studies on bone-marrow in chronic myeloid leukaemia. Lancet. 1963 Apr 20;1(7286):844–846. doi: 10.1016/s0140-6736(63)91620-9. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- WHANG J., FREI E., 3rd, TJIO J. H., CARBONE P. P., BRECHER G. THE DISTRIBUTION OF THE PHILADELPHIA CHROMOSOME IN PATIENTS WITH CHRONIC MYELOGENOUS LEUKEMIA. Blood. 1963 Dec;22:664–673. [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Osmond D. G. Blastogenic response of lymphocytes separated from bone marrow to allogeneic lymphoid cells in vitro. Immunology. 1971 Nov;21(5):767–779. [PMC free article] [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]


