Abstract
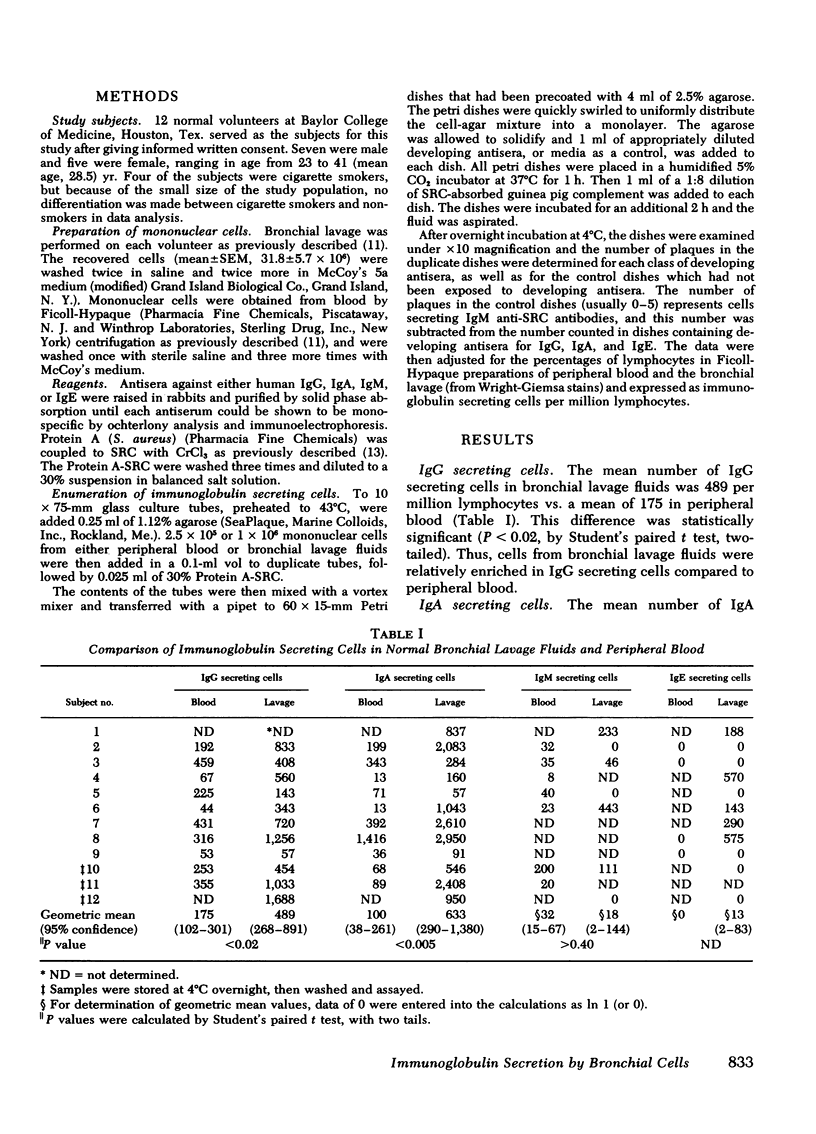
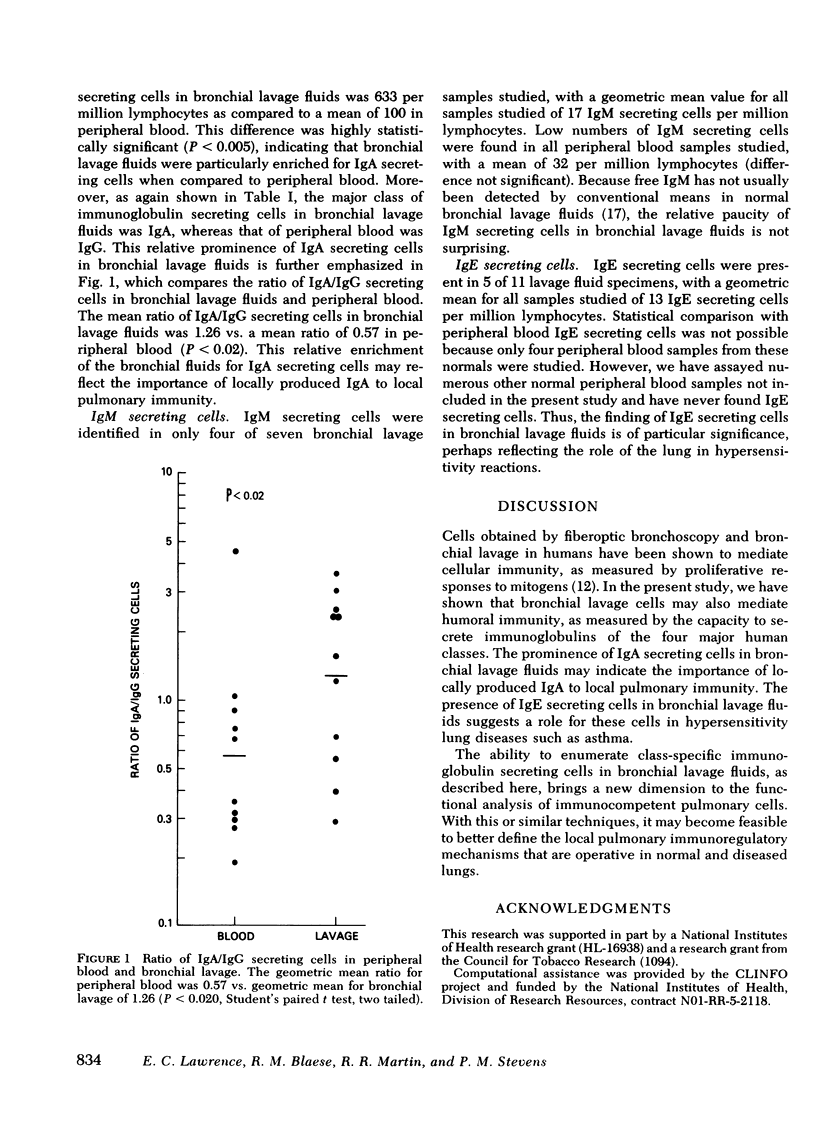
Immunoglobulin secreting cells were quantitated in the bronchial lavage fluids of 12 normal volunteers and compared with immunoglobulin secreting cells in peripheral blood, by a reverse hemolytic plaque assay. The mean number of cells secreting immunoglobulin (Ig)G in bronchial lavage fluids was 489 per million lymphocytes vs. a mean of 175 IgG secreting cells per million lymphocytes in peripheral blood (P < 0.02). The mean number of IgA secreting cells in bronchial lavage fluids was 633 per million lymphocytes as compared to 100 per million lymphocytes in peripheral blood (P < 0.005). Thus, compared to peripheral blood, cells from the lavage fluids were relatively enriched for both IgG and IgA secreting cells. However, IgA secreting cells were the major class of immunoglobulin secreting cells in bronchial lavage fluids, whereas IgG secreting cells predominated in peripheral blood. The prominence of IgA secreting cells in bronchial lavage fluids was further demonstrated by a mean ratio of IgA/IgG secreting cells in bronchial lavage fluids of 1.26 compared to a ratio in peripheral blood of 0.57 (P < 0.02). Cells secreting IgM were identified in only four of seven bronchial lavage fluid samples studied but in all peripheral blood samples. IgE secreting cells were not present in normal peripheral blood but could be demonstrated in 5 of 11 lavage fluid specimens. Thus, cells actively secreting immunoglobulins can be identified in the lower bronchial-alveolar tree of normal human subjects. Cells secreting IgG, IgA, or IgM may function in local lung defenses against infection; cells secreting IgE may contribute to hypersensitivity reactions in the lung.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., HUMPHREY J. H. Formation of specific antibodies and gamma-globulin in vitro; a study of the synthetic ability of various tissues from rabbits immunized by different methods. Biochem J. 1958 Feb;68(2):252–261. doi: 10.1042/bj0680252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Daniele R. P., Altose M. D., Rowlands D. T., Jr Immunocompetent cells from the lower respiratory tract of normal human lungs. J Clin Invest. 1975 Oct;56(4):986–995. doi: 10.1172/JCI108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Altose M. D., Rowlands D. T., Jr, Gorenberg D. J. Lymphocyte studies in asymptomatic cigarette smokers. A comparison between lung and peripheral blood. Am Rev Respir Dis. 1977 Dec;116(6):997–1005. doi: 10.1164/arrd.1977.116.6.997. [DOI] [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and mitogen-induced immunoglobulin-secreting cells in human peripheral blood: evaluation by a modified reverse hemolytic plaque assay. J Immunol. 1978 Jan;120(1):33–39. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hand W. L., Cantey J. R. Antibacterial mechanisms of the lower respiratory tract. I. Immunoglobulin synthesis and secretion. J Clin Invest. 1974 Feb;53(2):354–362. doi: 10.1172/JCI107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Kyselka L., Salmon S. E. Immunology of the lower respiratory tract. II. The plaque-forming response of canine lymphoid tissues to sheep erythrocytes after intrapulmonary or intravenous immunization. J Clin Invest. 1974 Aug;54(2):263–270. doi: 10.1172/JCI107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B., Salmon S. E. Immunology of the lower respiratory tract. Functional properties of bronchoalveolar lymphocytes obtained from the normal canine lung. J Clin Invest. 1973 Sep;52(9):2211–2217. doi: 10.1172/JCI107406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughter A. H., Martin R. R., Twomey J. J. Lymphoproliferative responses to antigens mediated by human pulmonary alveolar macrophages. J Lab Clin Med. 1977 Jun;89(6):1326–1332. [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Dale D. C., Wolff S. M. Changes in the composition of canine respiratory cells obtained by bronchial lavage following irradiation or drug immunosuppression. Proc Soc Exp Biol Med. 1976 Apr;151(4):756–761. doi: 10.3181/00379727-151-39301. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Warr G. A., Martin R. R., Holleman C. L., Criswell B. S. Classification of bronchial lymphocytes from nonsmokers and smokers. Am Rev Respir Dis. 1976 Jan;113(1):96–100. doi: 10.1164/arrd.1976.113.1.96. [DOI] [PubMed] [Google Scholar]


