Abstract
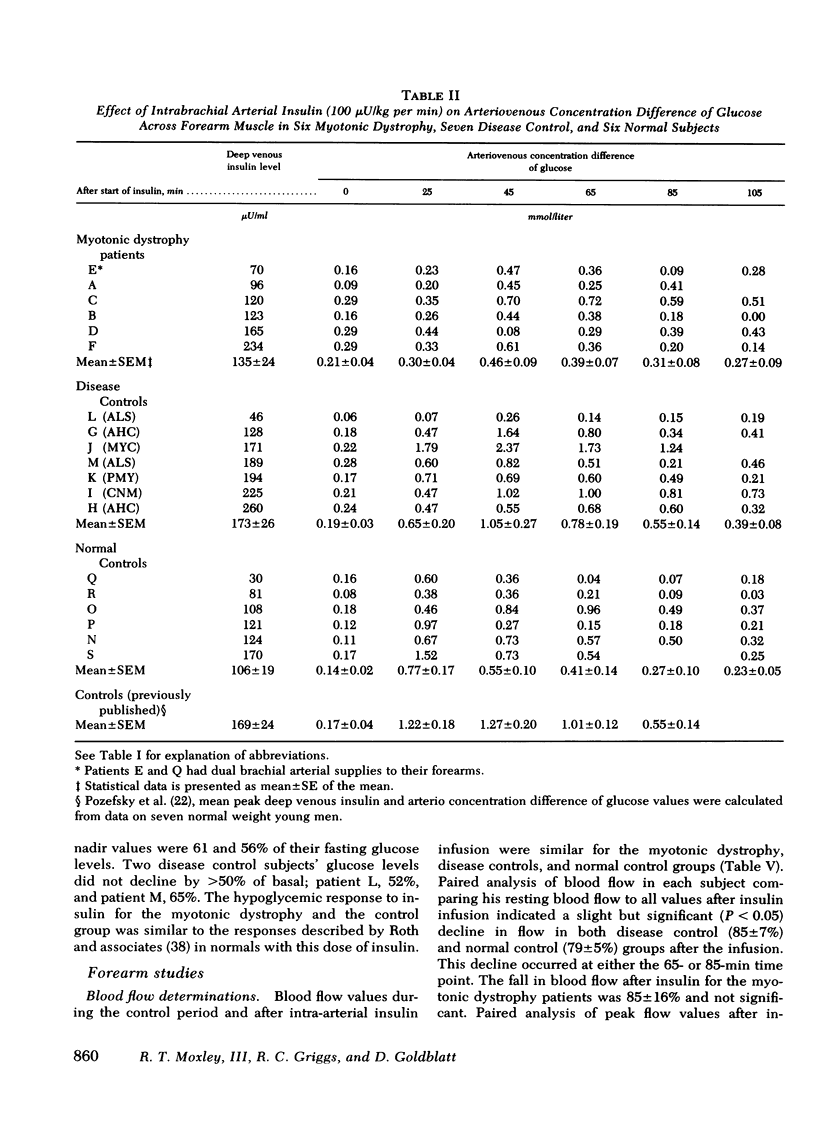
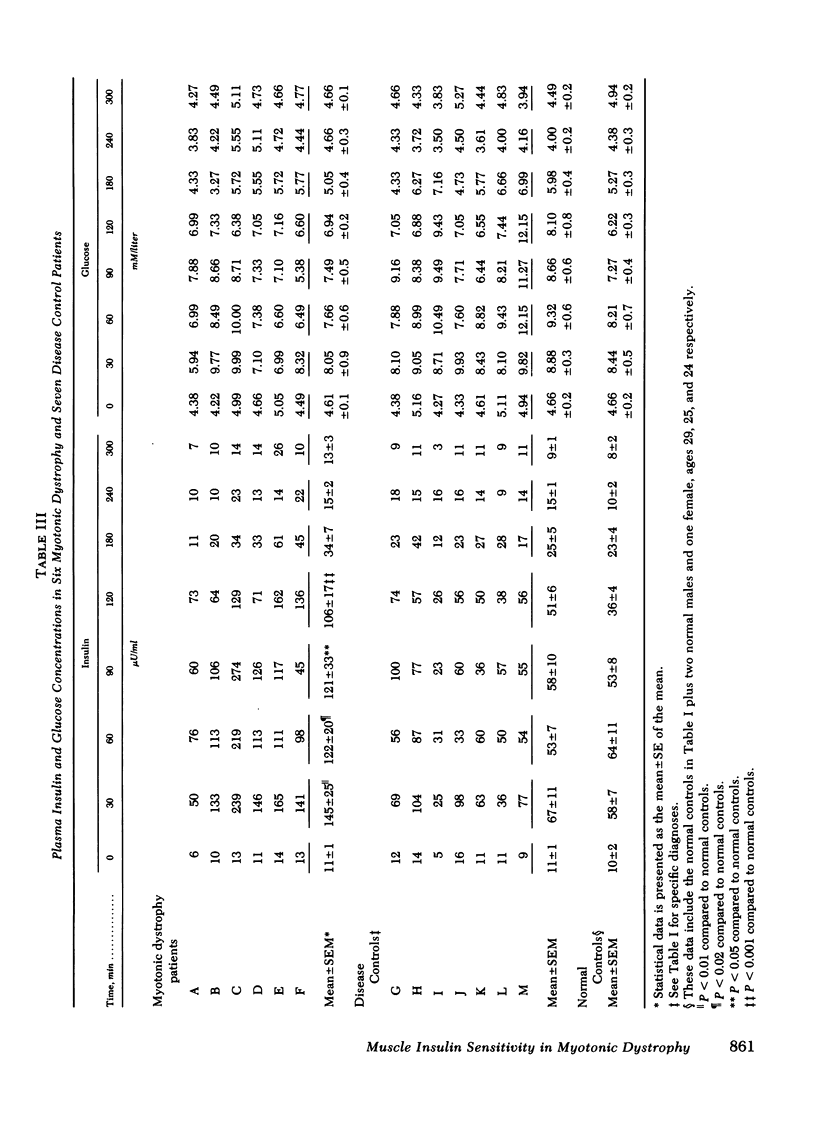
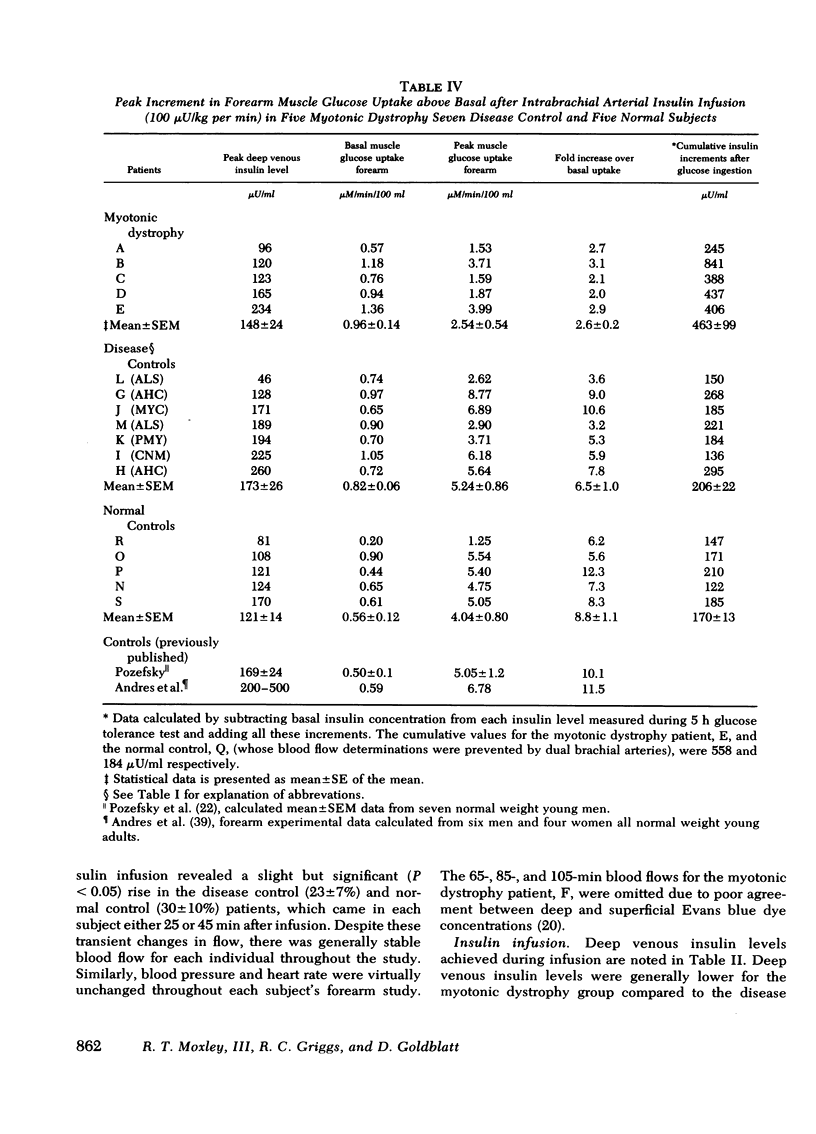
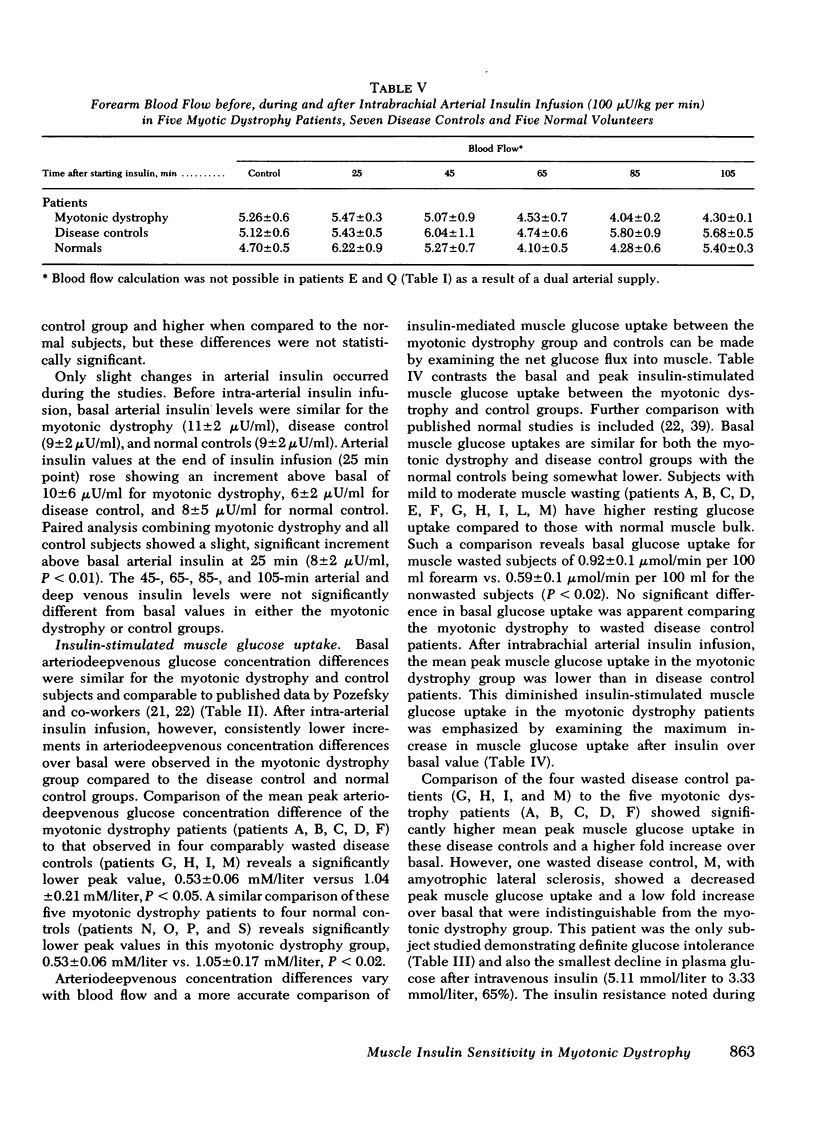
Previous studies of patients with myotonic dystrophy have demonstrated hyperinsulinism after glucose loading. This hyperinsulinism has been attributed by some investigators to tissue insulin resistance. We have directly studied insulin sensitivity of forearm muscle in patients having such hyperinsulinism. The effect of an intrabrachial arterial insulin infusion (100 mu U/kg per min) on glucose uptake was determined in six cases of myotonic dystrophy, six normal subjects, and in seven disease control subjects with myotonia or wasting from other disorders. There was no significant difference in insulin tolerance comparing myotonic dystrophy patients to the normal and disease control groups. Glucose tolerance and basal insulin levels were normal in the myotonic dystrophy patients, but hyperinsulinism occurred after glucose ingestion. After 25 min of intra-arterial insulin, the mean peak muscle glucose uptake in myotonic dystrophy was 2.54 +/- 0.54 mu mol/min per 100 ml forearm compared to 5.24 +/- 0.86 mu mol/min per 100 ml for disease controls (P is less than 0.05). Myotonic dystrophy patients showed a peak glucose uptake increment of only 2.6 +/- 0.2-fold over basal contrasted with the disease control value of 6.5 +/- 1.0-fold (P is less than 0.02) and the normal control value of 8.8 +/- 1.1-fold (P is less than 0.01). Thus, there was an absolute as well as a relative decrease in muscle insulin sensitivity in myotonic dystrophy patients compared to both control groups. The peak increments in arterio-superficial venous glucose concentration differences after insulin infusion were not significantly different comparing myotonic dystrophy and control groups. These data suggest that in myotonic dystrophy, there is insulin insensitivity of skeletal muscle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa J., Nuttall F. Q., Kennedy W., Goetz F. Plasma insulin in patients with myotonic dystrophy and their relatives. Medicine (Baltimore) 1974 Jul;53(4):307–323. doi: 10.1097/00005792-197407000-00004. [DOI] [PubMed] [Google Scholar]
- Bird M., Tzagournis M. Insulin secretion in myotonic dystrophy. Am J Med Sci. 1970 Dec;260(6):351–358. doi: 10.1097/00000441-197012000-00004. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Schröder G., Orndahl G. Carbohydrate and lipid metabolism in relation to body composition in myotonic dystrophy. Diabetes. 1973 Apr;22(4):238–242. doi: 10.2337/diab.22.4.238. [DOI] [PubMed] [Google Scholar]
- Bocek R. M., Basinger G. M., Beatty C. H. Comparison of glucose uptake and carbohydrate utilization in red and white muscle. Am J Physiol. 1966 May;210(5):1108–1111. doi: 10.1152/ajplegacy.1966.210.5.1108. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Price D. L., Watanabe C. K. Familial centronuclear myopathy. J Neurol Neurosurg Psychiatry. 1970 Oct;33(5):687–693. doi: 10.1136/jnnp.33.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Engel W. K. The histographic analysis of human muscle biopsies with regard to fiber types. 3. Myotonias, myasthenia gravis, and hypokalemic periodic paralysis. Neurology. 1969 May;19(5):469–477. doi: 10.1212/wnl.19.5.469. [DOI] [PubMed] [Google Scholar]
- Davies A. S., Gunn H. M. Histochemical fibre types in the mammalian diaphragm. J Anat. 1972 May;112(Pt 1):41–60. [PMC free article] [PubMed] [Google Scholar]
- FORBES G. B., HURSH J. B. AGE AND SEX TRENDS IN LEAN BODY MASS CALCULATED FROM K40 MEASUREMENTS: WITH A NOTE ON THE THEORETICAL BASIS FOR THE PROCEDURE. Ann N Y Acad Sci. 1963 Sep 26;110:255–263. doi: 10.1111/j.1749-6632.1963.tb17090.x. [DOI] [PubMed] [Google Scholar]
- Fajans S. S. What is diabetes? Definition, diagnosis, and course. Med Clin North Am. 1971 Jul;55(4):793–805. doi: 10.1016/s0025-7125(16)32476-2. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Fineberg S. E., Merimee T. J. Effects of comparative perfusions of equimolar, single component insulin and proinsulin in the human forearm. Diabetes. 1973 Sep;22(9):676–686. doi: 10.2337/diab.22.9.676. [DOI] [PubMed] [Google Scholar]
- Forbes G. B., Bruining G. J. Urinary creatinine excretion and lean body mass. Am J Clin Nutr. 1976 Dec;29(12):1359–1366. doi: 10.1093/ajcn/29.12.1359. [DOI] [PubMed] [Google Scholar]
- Forbes G. B., Schultz F., Cafarelli C., Amirhakimi G. H. Effects of body size on potassium-40 measurement in the whole body counter (tilt-chair technique). Health Phys. 1968 Nov;15(5):435–442. doi: 10.1097/00004032-196811000-00004. [DOI] [PubMed] [Google Scholar]
- Goden P., Griggs R. C., Nissley S. P., Roth J., Engel W. K. Studies of plasma insulin in myotonic dystrophy. J Clin Endocrinol Metab. 1969 May;29(5):684–690. doi: 10.1210/jcem-29-5-684. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Heymsfield S. B., Bethel R. A., Rudman D. Hyperresponsivness of patients with clinical and premyopathic myotonic dystrophy to human growth hormone. J Clin Endocrinol Metab. 1977 Jul;45(1):147–158. doi: 10.1210/jcem-45-1-147. [DOI] [PubMed] [Google Scholar]
- Huff T. A., Horton E. S., Lebovitz H. E. Abnormal insulin secretion in myotonic dystrophy. N Engl J Med. 1967 Oct 19;277(16):837–841. doi: 10.1056/NEJM196710192771602. [DOI] [PubMed] [Google Scholar]
- Huff T. A., Lebovitz H. E. Dynamics of insulin secretion in myotonic dystrophy. J Clin Endocrinol Metab. 1968 Jul;28(7):992–998. doi: 10.1210/jcem-28-7-992. [DOI] [PubMed] [Google Scholar]
- Karpati G., Carpenter S., Nelson R. F. Type I muscle fibre atrophy and central nuclei. A rare familial neuromuscular disease. J Neurol Sci. 1970 May;10(5):489–500. doi: 10.1016/0022-510x(70)90027-4. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. Observations on endocrine function in dystrophia myotonica. Brain. 1959 Jun;82:221–231. doi: 10.1093/brain/82.2.221. [DOI] [PubMed] [Google Scholar]
- Nuttall F. Q., Barbosa J., Gannon M. C. The glycogen synthase system in skeletal muscle of normal humans and patients with myotonic dystrophy: effect of glucose and insulin administration. Metabolism. 1974 Jun;23(6):561–568. doi: 10.1016/0026-0495(74)90084-5. [DOI] [PubMed] [Google Scholar]
- PARK C. R., JOHNSON L. H. Effect of insulin on transport of glucose and galactose into cells of rat muscle and brain. Am J Physiol. 1955 Jul;182(1):17–23. doi: 10.1152/ajplegacy.1955.182.1.17. [DOI] [PubMed] [Google Scholar]
- Poffenbarger P. L., Bozefsky T., Soeldner J. S. The direct relationship of proinsulin-insulin hypersecretion to basal serum levels of cholesterol and triglyceride in myotonic dystrophy. J Lab Clin Med. 1976 Mar;87(3):384–396. [PubMed] [Google Scholar]
- Polgar J. G., Bradley W. G., Upton A. R., Anderson J., Howat J. M., Petito F., Roberts D. F., Scopa J. The early detection of dystrophia myotonica. Brain. 1972;95(4):761–766. doi: 10.1093/brain/95.4.761. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozefsky T., Santis M. R., Soeldner J. S., Tancredi R. G. Insulin sensitivity of forearm tissues in prediabetic man. J Clin Invest. 1973 Jul;52(7):1608–1615. doi: 10.1172/JCI107338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozefsky T., Tancredi R. G., Moxley R. T., Dupre J., Tobin J. D. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes. 1976 Feb;25(2):128–135. doi: 10.2337/diab.25.2.128. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSONSA Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963 May 31;140(3570):987–988. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Griggs R. C., Moxley R. T. Acetazolamide-induced weakness in paramyotonia congenita. Ann Intern Med. 1977 Feb;86(2):169–173. doi: 10.7326/0003-4819-86-2-169. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Muscle membrane protein kinase in myotonic muscular dystrophy. Nature. 1974 Jul 19;250(463):245–247. doi: 10.1038/250245a0. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Protein kinase activity in erythrocyte ghosts of patients with myotonic muscular dystrophy. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1855–1859. doi: 10.1073/pnas.70.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Chyatte S. B., Patterson J. H., Gerron G. G., O'Beirne I., Barlow J., Ahmann P., Jordan A., Mosteller R. C. Observations on the responsiveness of human subjects to human growth hormone. Effects of endogenous growth hormone deficiency and myotinic dystrophy. J Clin Invest. 1971 Sep;50(9):1941–1949. doi: 10.1172/JCI106686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Tevaarwerk G. J., Hudson A. J. Carbohydrate metabolism and insulin resistance in myotonia dystrophica. J Clin Endocrinol Metab. 1977 Mar;44(3):491–498. doi: 10.1210/jcem-44-3-491. [DOI] [PubMed] [Google Scholar]
- Walsh J. C., Turtle J. R., Miller S., McLeod J. G. Abnormalities of insulin secretion in dystrophia myotonica. Brain. 1970;93(4):731–742. doi: 10.1093/brain/93.4.731. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. ROLES OF INSULIN AND GROWTH HORMONE, BASED ON STUDIES OF FOREARM METABOLISM IN MAN. Medicine (Baltimore) 1963 Nov;42:385–402. doi: 10.1097/00005792-196311000-00002. [DOI] [PubMed] [Google Scholar]
- Zellweger H., Ionasescu V. Myotonic dystrophy and its differential diagnosis. Acta Neurol Scand Suppl. 1973;55:5–28. [PubMed] [Google Scholar]


