Abstract
Improvements in osteoconduction of implant biomaterials require focusing on the bone-implant interface, which is a complex multifactorial system. Surface topography of implants plays a crucial role at this interface. Nanostructured surfaces have been shown to promote serum protein adsorption and osteoblast adhesion when compared to microstructured surfaces for bone-implant materials. We studied the influence of the serum proteins fibronectin and vitronectin on the attachment and proliferation of osteoblasts onto nanostructured titania surfaces. Human fetal osteoblastic cells hFOB 1.19 were used as model osteoblasts and were grown on nanoporous TiO2 templates, using Ti6Al4V and commercially pure Ti substrates as controls. Results show a significant increase in cell proliferation on nanoporous TiO2 over flat substrates. Initial cell attachment data exhibited a significant effect by either fibronectin or vitronectin on cell adhesion at the surface of any of the tested materials. In addition, the extent of cell adhesion was significantly different between the nanoporous TiO2 and both Ti6Al4V and commercially pure Ti substrates, with the first showing the highest surface coverage. There was no significant difference on osteoblast attachment or proliferation between the presence of fibronectin or vitronectin using any of the material substrates. Taken together, these results suggest that the increase in osteoblast attachment and proliferation shown on the nanoporous TiO2 is due to an increase in the adsorption of fibronectin and vitronectin because of the higher surface area and to an enhanced protein unfolding, which allows access to osteoblast binding motifs within these proteins.
Keywords: nanoporous TiO2, hFOB 1.19, protein adsorption, vitronectin, fibronectin
1. INTRODUCTION
Osteoconduction is the growth of bony tissue into the structure of an implant biomaterial.1 Improvements in the osteoconductive capacity of a biomaterial requires focusing efforts in the bone-implant interface, which is complex and involves numerous factors, e.g., mechanical loading, implant material-related factors, such as composition, topography, and surface energy, and patient variables, such as bone quantity and quality.2 Since osteoconduction involves the direct interaction between bone cells and the biomaterial, the understanding of the adhesion of osteoblasts forming the bone-implant interface will pave the way to the design of new and efficient implant biomaterials.
Commercially pure titanium (cpTi) and its alloys (e.g., Ti6Al4V and Ti6Al7Nb) are widely used for the fabrication of prosthetics with the former being commonly used for load bearing devices such as artificial hip joint stems. Their use as biomaterials offer attractive features which include excellent corrosion resistance, biocompatibility and good strength-to-weight ratio.3 The induction of corrosion resistance and biocompatibility are related to the presence of an oxide (passive) layer, which prevents the formation of fibrous tissue around the implant, and creates direct contact to the bone tissue. In Ti6Al4V the mixed oxide layer consists of mainly TiO2 with 6–8 at% Al and less than 0.1 at% V in the outermost atomic layers of the oxide.4,5 The percentage of area coverage by each of these oxides is strongly dependent on the surface composition and processing conditions. This oxide layer also has a significant effect on the Ca/P ratio in the calcium phosphate film deposited when compared to pure titanium alloy during mineralization.6,7 In comparison with cpTi, the calcium phosphates formed on Ti6Al4V had a Ca/P ratio less similar to that of naturally-occurring hydroxyapatite, which can be attributed to the presence of regions consisting of aluminum oxide.6,7,8
An important part of the osseointegration is the osteoblast adhesion process which involves protein adsorption, cell interaction with the adsorbed proteins, cell attachment and spreading on the implant surface.9 In cell culture, protein adsorption on flat surfaces (e.g., tissue culture polystyrene) occurs nearly instantaneously forming a 2–5 nm layer through molecular-scale interactions with the substrate. Such proteins are part of the serum which is used to supplement the cell culture media and include extracellular matrix (ECM) proteins such as collagen, thrombospondin, fibronectin, vitronectin, and osteopontin. Among these, fibronectin and vitronectin are of particular interest for osseointegration since they induce the reorganization of actin microfilaments promoting cell adhesion and spreading, which in turn affects cell morphology and migration. 10–13
When cells come in contact with this adsorbed protein layer (i.e., ~ 5 min after seeding), they attach through non-specific physicochemical interactions, such as ionic and van der Walls forces. This is followed by recognition of cell binding motifs on these proteins which is mediated by integrins, a widely expressed family of transmembrane adhesion receptors, consisting of α and β heterodimers, which bind to specific amino acid sequences, such as the RGD cell binding domain.14,15 Upon binding to their ligand, integrins rapidly associate with the actin network of the cytoskeleton and cluster together to form focal adhesions, which are discrete complexes that contain structural and signaling molecules 16,17 and function as structural links between the cytoskeleton and ECM to mediate cell adhesion and migration. In conjunction with growth-factor receptors, focal adhesions activate signaling pathways such as MAPK and JNK which regulate transcription factor activity and direct cell proliferation and other functions.18–20 At later times, cell attachment is strengthened by synthesis and deposition of additional ECM proteins promoting a stronger binding.9 The layer of adsorbed proteins mediate subsequent interactions with cells from the surrounding tissues, promoting cell functions pertinent to new tissue formation, which lead to integration and stabilization of the implant.10,21
The chemical and physical characteristics of the material surface affect the amount, distribution, density, conformation and orientation of adsorbed proteins.22–24 Although all aspects and the underlying mechanisms of surface-protein interactions are not well understood, it is known that the material surface chemistry is a determining factor. In addition to surface composition (i.e., biomaterial chemical composition and proteins adsorbed), surface topography also plays an important role in osseointegration. For instance, an increasing number of studies demonstrate that nanoscale topographies significantly enhance cell functions leading to improved osseointegration compared to conventional micron-structured surfaces.25–28 Recent interest in nanoporous TiO2 templates as alternatives to improve on Ti-based implants have shown promise by improving osteoblast attachment and proliferation.29–32 These nanoporous templates can be used to modify implants by RF sputtering followed by anodization.33 Despite all the work focused on the biomaterial-tissue interface, an understanding on how biomaterial surface properties and the adsorbed protein layer affect cell behavior is still lacking. While investigations have found enhanced protein adsorption on nanostructured surfaces34–36 very few studies have focused on its effect on cell function.37 In this work, we have fabricated nanostructured TiO2 substrates using a simple anodization process,30,38 to study the influence of the serum proteins fibronectin (FN) and vitronectin (VN), and nanotopography on osteoblast cell attachment and proliferation using the human fetal osteoblast cell line hFOB 1.19. Simple methods to improve the osteoconductive capacity of biomaterials will have a strong impact on the orthopedic implant industry.
2. MATERIALS AND METHODS
2.1. Nanoporous TiO2 Fabrication
Nanoporous TiO2 templates were fabricated by anodization of titanium foil (Alfa Aesar) under a constant voltage of 60 V and in potassium fluoride (0.05M in 10% deionized (DI) water/90% glycerol) solution. After 15 h of anodization, nanotubes 125 nm in diameter and 2.5mm in length were achieved as previously reported.38
2.2.Characterization
Scanning electron microscopy (SEM) was used to characterize the surface morphology of the nanoporous TiO2 templates and the Ti6Al4V and cpTi substrates using a Field Emission Scanning Electron Microscope Supra 35 (Zeiss). The discs were analyzed in the “as-received” condition and without the use of coatings. The equipment allows analysis of features in the nanometer range using low accelerating voltage (3kV).
2.3. Cell culture
Human osteoblast cell line hFOB 1.19 (CRL-11372, ATCC, Manassas, VA,USA) was cultured in 90% Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 Ham, 1:1 (DMEM) (Sigma-Aldrich, St. Louis, MO) with 2.5mM L-Glutamine and 15mM Hepes, without phenol red, supplemented with 0.3 mg/ml G418 (Sigma-Aldrich, St. Louis, MO) and 10% Fetal Bovine Serum (FBS) (Sigma-Aldrich, St. Louis, MO). Cells were cultured at 33.5°C and 5% CO2. At confluence, cells were trypsinated and seeded at a final concentration of 1x104 cells/cm2 onto the substrates.
2.4. Substrate preparation for cell culture experiments
Metal rods 1cm in diameter by 30 cm in length of Ti6Al4V and cpTi were obtained from a commercial source, which were cut into disks 0.1 cm in thickness. Silicon carbide paper of grits 240, 320, 400 and 600 were used sequentially to grind the Ti6Al4V and cpTi metallic surfaces. After grinding, a solution of Al2O3 (alumina particles 5μm and 3μm in size) was used as the polishing agent. The substrates were further polished by a polycrystalline diamond suspension with particle size of 3μm. Nanoporous TiO2 templates used were 1cm-by-1cm in size and, Ti6Al4V and cpTi substrates were 1.0 cm in diameter and 0.1 cm in thickness (0.5 cm2 area). All substrates were incubated for 30 min in 0.53 mM EDTA/D-PBSA, washed with distilled DI water, scrapped using a plastic cell scraper, washed with absolute alcohol and washed again with distilled DI water. Thereafter the discs and controls were sterilized by autoclaving at 15 psi and 121°C and placed in sterile 6-well plates (Corning, NY).
The nanoporous TiO2 templates and the Ti6Al4V and cpTi substrates were incubated with either 10% FBS, or serum levels of FN (20 μg/mL) of VN (2 μg/mL) for 24 h at 37°C. After this time period, each substrate was gently rinsed with PBS at room temperature just before seeding with cells.
2.5. Cell adhesion experiments and imaging
The hFOB 1.19 cells were cultured on substrates at a density of 104 cells/ml and incubated for 6 hours, at 33.5°C and 5% CO2. Substrates were washed twice with serum-free medium and once with D-PBSA. Cells were fixed using methanol at 4°C for 10 min and washed three times with D-PBSA. Non-specific staining was blocked by incubation in blocking solution (1% BSA in D-PBSA) for 1h at room temperature. The blocking solution was removed and the cells were permeabilized with 0.5% triton X-100 at 4oC for 5 min, washed with D-PBSA and incubated with Phalloidin-TRITC (Sigma-Aldrich, St. Louis, MO) at 10 μg/ml. After 1h at room temperature of incubation, substrates were washed with D-PBSA and followed by the addition of 0.8 mg/ml DAPI (Research organics, Cleveland, OH), for 15min at 37oC. After washing again the substrates with D-PBSA, they were fixed with mounting media and stored at 4°C in the dark. Images were captured using an inverted confocal laser scanning microscope (CLSM) (FluoView 300, Olympus, USA) equipped with 405nm, 543nm Green HeNe and 633nm Red HeNe lasers. Phalloidin-TRITC has a peak excitation at 540–545nm and a peak emission at 570–573nm, while DAPI has a peak excitation at 345–350nm and a peak emission at 455–470nm.
2.6. Cell Viability
Viability of the cells cultured on the substrates were assessed at 6 hours of culture using the commercially available LIVE/DEAD viability/cytotoxicity assay (Invitrogen) and following manufacturer’s instructions. Stained cells were visualized in situ using fluorescent microscopy. Live cells appear green (excitation: 494 nm; emission: 517 nm), while dead cells appear red (excitation: 528 nm; emission: 617 nm). Micrographs (taken at twenty five random fields of view) were obtained and used to quantify the number of live and dead cells per substrate tested; percent cell viability on each substrate was thus obtained. These experiments were conducted in triplicate.
2.7. Proliferation rate of hFOB 1.19 cells
Cell proliferation was measured at 1, 3 and 7 days on the nanoporous TiO2 templates and the Ti6Al4V and cpTi substrates. Cells were cultured on substrates at a density of 104 cells/cm2 and incubated at 33.5°C and 5% CO2, following labeling with Phalloidin-TRICT (Sigma-Aldrich, St. Louis, MO) at 10 μg/ml. Cells were enzymatically released (1 mM EDTA, 1.3 mg/mL collagenase and 0.25% trypsin; Gibco) and counted using a hemocytometer.
2.8. Statistical analysis
The Student’s t-test (two-tailed) was used to evaluate the significance of changes in cell proliferation between control and experimental groups. A probability value of p<0.05 was considered statistically significant. All data were analyzed using GraphPad Prism 5.
3. RESULTS AND DISCUSSION
Improvements in the osteoconductive capacity of implant biomaterials require the study of how important implant biomaterial-related factors, such as topography and composition promote osteoblast attachment. Nanostructured materials have shown increased cell attachment over microstructured or smooth surfaces.39,40 An alternative for fabricating nanostructures at the surface of implant materials is the use of an anodization process. 29–32 This is an attractive alternative since it can be easily applied to existing implants without affecting current fabrication processes.41 In addition to nanotopography, biomaterial surfaces modified with ECM proteins or cell-binding motifs have also shown an increase in cell attachment.42,43 To study the effect of these parameters we have fabricated nanoporous TiO2 substrates and measured the influence of the FN and VN on osteoblast cell attachment and proliferation on these nanostructured substrates using the human fetal osteoblastic cell line hFOB 1.19.
Figure 1 shows SEM images of cpTi, Ti6Al4V substrates and nanoporous TiO2 templates. The nanoporous TiO2 with pore diameter of approximately 125 nm and length of 200 μm were prepared using an anodization voltage of 60V for 15 h. It can be seen that the nonopore distribution is uniform over the substrate. In addition, anodizing conditions can be tuned (e.g., voltage, electrolyte, and pH) to optimize parameters such as pore diameter and length to maximize osteoinduction.44
Figure 1.
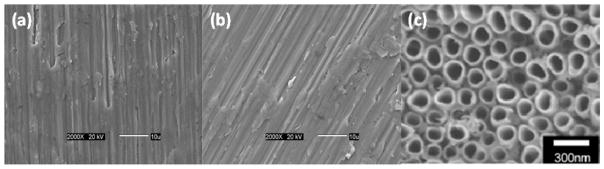
SEM images of (a) cpTi substrates, (b) Ti6Al4V substrates and c) nanoporous TiO2 templates. Bars (a) and (b) 10 μm, and (c) 300 nm.
The effect of adsorbed FN or VN on the adhesion of hFOB 1.19 cells on fabricated nanoporous TiO2 templates, Ti6Al4V and cpTi substrates after 6 h of seeding is shown in Figure 2. During this time, cells reach an equilibrium adherence level where they spread on the surface and depend on receptor-mediated binding.45 For all three substrates the presence of proteins (i.e., FN, VN or FBS) had a significant (p < 0.05) effect on adhesion. In addition, difference in cell adhesion between FN or VN and FBS were significant (p < 0.05) in the case of the Ti6Al4V and cpTi substrates, but not for the nanoporous TiO2 templates. In both the Ti6Al4V and cpTi substrates, FBS decreased the number of cells attached to the substrate, which could be explained by competitive adsorption of other proteins in the serum that are either in larger quantities or with a higher affinity for the substrate.46,47 Also, these two substrates showed no significant difference in cell adhesion between them in the presence of FBS or when proteins were not present. Different from these two substrates, nanoporous TiO2 templates had no significant difference between FN, VN or FBS. It has been shown that nanostructured materials allow for an increase in protein unfolding39,40 and adsorbed protein density.48 For instance, both FN and VN must unfold on the nanoporous surface to allow access to their otherwise hidden cell-binding RGD tripeptide sites.39,49 Thus, nanoporous TiO2 templates could allow higher cell-binding by providing a higher density of cell-binding motifs over the other two substrates. This was shown by the significantly enhanced cell adhesion on the nanoporous TiO2 templates over both the Ti6Al4V and cpTi substrates. In addition to improving protein adsorption and unfolding to allow cells access to the binding motifs, nanopores can also be used as reservoirs of growth factors which affect osteoprogenitor cell proliferation and differentiation such as insulin-like growth factors (IGFs), transforming growth factor-βs (TGFβs), and bone morphogenetic proteins (BMPs).50 In a recent study by our group we presented an alumina nanopore template for the sustained release of the model drug doxorubicin over several days.38 It was observed that after 6 h of culture cell viability ranged between 93% and 100% on all the substrates (Data not shown).
Figure 2.
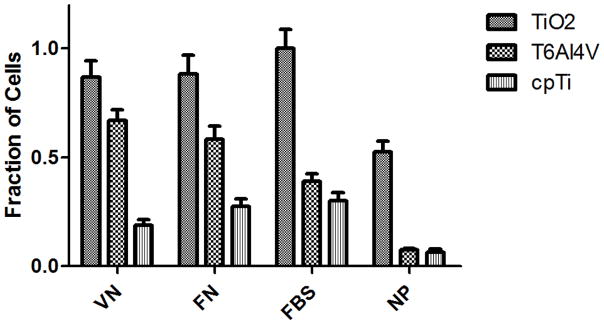
Effect of adsorbed protein on hFOB 1.19 adhesion after 6 h of being seeded onto nanoporous TiO2 templates, Ti6Al4V and cpTi substrates. Data is normalized to the highest number of cells on a template. Bars = mean + SEM with n = 25.
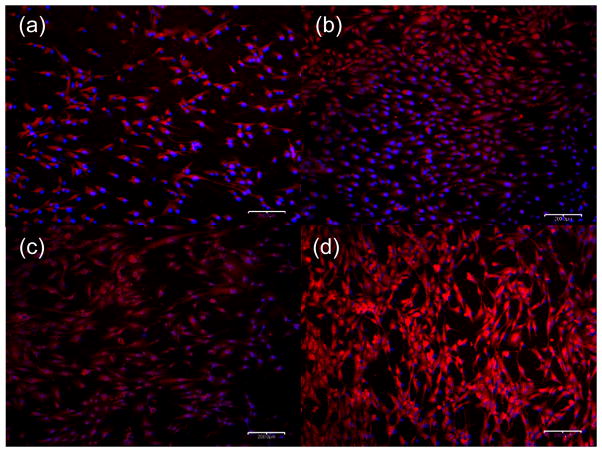
To assess cell morphology, hFOB 1.19 cells were analyzed by fluorescence microscopy after 3 days in culture on Ti6Al4V, cpTi, and nanoporous TiO2 that were pre-treated with or without VN (Figure 3). Images similar to the VN-treated surfaces (figures 3a and 3b) were obtained when these were treated with FN and are not shown. In addition, results using cpTi were not shown because of the low cell coverage obtained when compared to the Ti6Al4V substrates. Figure 3 clearly shows that the presence of proteins increases cell attachment on either substrate. Furthermore, Figure 3d shows the formation of cell clusters, which result from cell spreading and interaction and eventually drives their differentiation. This is expected since after three days hFOB 1.19 cells are in full exponential growth at which time cells start to cluster in a manner proportional to proliferation.51 Thus, nanoporous TiO2 templates in the presence of cell-binding proteins promote a more proliferative surface coverage over either Ti6Al4V or cpTi substrates.
Figure 3.
Fluorescence microscopy images (10x) of hFOB 1.19 cells on Ti6Al4V substrates without and with VN treatment ((a) and (c), respectively) and nanoporous TiO2 templates without and with VN treatment ((b) and (d), respectively).Cells were stained with phalloidin-TRITC (red) and DAPI (blue) to see the F-actin cytoskeleton and nuclei, respectively. Bar = 200 μm.
To quantify the results shown in Figure 3, hFOB cell proliferation was measured at 1, 3 and 7 days of culture in the presence of 10% FBS. Figure 4 shows a much faster hFOB cell proliferation rate using the nanoporous TiO2 template over the Ti6Al4V or cpTi substrates, confirming the previous results shown in Figure 3. The results for each of the biomaterials were significantly different from each other (p < 0.05).
Figure 4.
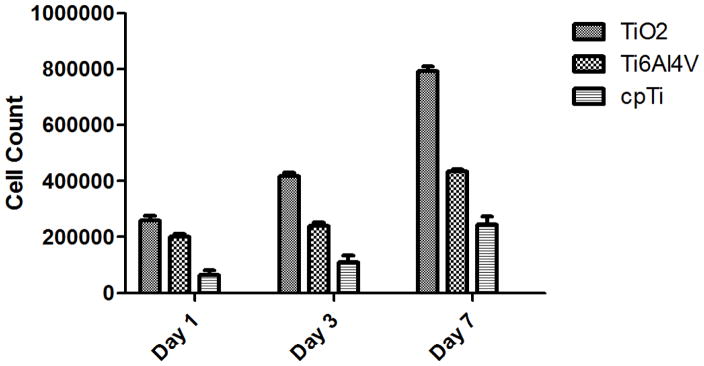
hFOB 1.19 cell proliferation on nanoporous TiO2 templates, Ti6Al4V and cpTi substrates for up to 7 days of culture in the presence of 10% FBS. Nanoporous templates show about 40% higher cell proliferation after 7 days of culture than cpTi substrates (p < 0.05). Bars = mean + SEM with n = 25.
4. CONCLUSION
Simple methods to improve the osteoconductive capacity of biomaterials will have a strong impact on the orthopedic implant industry by providing implants which can provide better osseointegration. In this work, we have shown a strong effect on ostreoblast proliferation by the ECM proteins VN an FN onto nanoporous TiO2 templates over the standard Ti6Al4V and cpTi. These nanopores can me generated on Ti-based implant biomaterials using simple anodization, which can be implemented on existing implants. Because of the increased surface area, nanoporous TiO2 templates are able to adsorb more protein than flat microstructured materials such as the Ti6Al4V or cpTi allowing improved cell attachment and proliferative capacity. In addition, these nanostructured templates seem to allow osteoblast-binding ECM proteins to unfold and permit access to their cell-binding motifs, such as the RGD tripeptide. Further studies are under way to study the conformational changes that occur upon FN and VN adsorption to the nanoporous TiO2 templates.
Acknowledgments
Funding from NSF and NIH grant numbers 0420604 and 1SC2GM089642, respectively, at UPRM and NSF-DGE-0965843 at Northeastern University is gratefully acknowledged. MAV and DMRC were supported through a NIH-RISE program grant fellowship R25GM088023.
References
- 1.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10:S96. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28:198. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Bränemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 4.Roessler S, Zimmermann R, Scharnweber D, Werner C, Worch H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Coll Surf B: Biointerfaces. 2002;26:387. [Google Scholar]
- 5.Gabriel BL, Gold J, Gristina AG, Kasemo B, Lausmaa J, Harrer C, Myrvik QN. Site-specific adhesion of Staphylococcus epidermidis (RP12) in Ti-Al-V metal systems. Biomaterials. 1994;15:628. doi: 10.1016/0142-9612(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 6.Hanawa T. Titanium and its oxide film: a substrate for the formation of apatite. In: Davies JE, editor. The bone-biomaterial interface. University of Toronto press; Toronto: 1991. p. 49. [Google Scholar]
- 7.Hanawa T, Asami K, Asaoka K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J Biomed Mater Res. 1998;40:530. doi: 10.1002/(sici)1097-4636(19980615)40:4<530::aid-jbm3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Hanawa T, Ota M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials. 1991;12:767. doi: 10.1016/0142-9612(91)90028-9. [DOI] [PubMed] [Google Scholar]
- 9.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 10.Scotchford CA, Ball M, Winkelmann M, Vörös J, Csucs C, Brunette DM, Danuser G, Textor M. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions. II: Protein adsorption and early cell interactions. Biomaterials. 2003;24:1147. doi: 10.1016/s0142-9612(02)00488-x. [DOI] [PubMed] [Google Scholar]
- 11.Steele JG, McFarland C, Dalton BA, Johnson G, Evans MD, Howlett CR, Underwood PA. Attachment of human bone cells to tissue culture polystyrene and to unmodified polystyrene: the effect of surface chemistry upon initial cell attachment. J Biomater Sci Polym Ed. 1993;5:245. doi: 10.1163/156856293x00339. [DOI] [PubMed] [Google Scholar]
- 12.Howlett CR, Evans MD, Walsh WR, Johnson G, Steele JG. Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials. 1994;15:213. doi: 10.1016/0142-9612(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 13.Kilpadi KL, Chang PL, Bellis SL. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res. 2001;57:258. doi: 10.1002/1097-4636(200111)57:2<258::aid-jbm1166>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs and cbfa1. Endocr Rev. 2000;21:393. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 15.García AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005;26:7525. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 16.De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 17.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 18.Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of notch, Wnt, and transforming growth factor-beta signaling pathways in bone morphogenic protein 2-induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. Biol Chem. 2004;279:37704. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 21.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 22.Eckert R, Jeney S, Hörber JK. Understanding intercellular interactions and cell adhesion: lessons from studies on protein-metal interactions. Cell Biol Int. 1997;21:707. doi: 10.1006/cbir.1997.0215. [DOI] [PubMed] [Google Scholar]
- 23.Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127:8168. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 24.Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007;18:630. doi: 10.1111/j.1600-0501.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 25.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanostructured metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;19:4731. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Dalby MJ, McCloy D, Robertson M, Wilkinson CD, Oreffo RO. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials. 2006;27:1306. doi: 10.1016/j.biomaterials.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Meirelles L, Currie F, Jacobsson M, Albrektsson T, Wennerberg A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int J Oral Maxillofac Implants. 2008;23:641. [PubMed] [Google Scholar]
- 28.Ruck T, Porter JR, Allam NK, Feng X, Grimes CA, Popat KC. Nanostructured tantala as a template for enhanced osseointegration. Nanotechnology. 2009;20:045102. doi: 10.1088/0957-4484/20/4/045102. [DOI] [PubMed] [Google Scholar]
- 29.Yao C, Perla V, McKenzie JL, Slamovich EB, Webster TJ. Anodized Ti and Ti6Al4V Possessing Nanometer Surface Features Enhances Osteoblast Adhesion. J Biomed Nanotechnol. 2005;1:68. [Google Scholar]
- 30.Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28:3188. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Cho KP, Chung YS, Kim OS, Chung SS, Lee KK, Lee DJ, Lee KM, Kim YJ. The effect of nanotubular titanium surfaces on osteoblast differentiation. J Nanosci Nanotechnol. 2010;10:3581. doi: 10.1166/jnn.2010.2235. [DOI] [PubMed] [Google Scholar]
- 32.Yang JS, Vang MS, Uhm SW, Chung YS, Lee KK, Lee DJ, Chung HJ, Kim YJ. Response of fetal rat calvarial cells to nanotubular titanium oxide surface. J Nanosci Nanotechnol. 2011;11:1807. doi: 10.1166/jnn.2011.3413. [DOI] [PubMed] [Google Scholar]
- 33.Fröjd V, Linderbäck P, Wennerberg A, Chávez de Paz L, Svensäter G, Davies JR. Effect of nanoporous TiO2 coating and anodized Ca2+ modification of titanium surfaces on early microbial biofilm formation. BMC Oral Health. 2011;11:8. doi: 10.1186/1472-6831-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai K, Bossert J, Jandt K. Does nanometre scale topography of titanium influence protein adsorption and cell proliferation? Col Surf B. 2006;49:136. doi: 10.1016/j.colsurfb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Chastain SR, Kundu AK, Dhar S, Calvert JW, Putnam AJ. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78:73. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 36.González-García C, Sousa SR, Moratal D, Rico P, Salmerón-Sánchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organization. Colloids Surf B Biointerfaces. 2010;77:181. doi: 10.1016/j.colsurfb.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Garnes M, González-García C, Moratal D, Rico P, Salmerón-Sánchez M. Fibronectin distribution on demixed nanoscale topographies. Int J Artif Organs. 2011;34:54. doi: 10.5301/ijao.2011.6316. [DOI] [PubMed] [Google Scholar]
- 38.Gultepe E, Nagesha D, Casse BD, Banyal R, Fitchorov T, Karma A, Amiji M, Sridhar S. Sustained drug release from non-eroding nanoporous templates. Small. 2010;6:213. doi: 10.1002/smll.200901736. [DOI] [PubMed] [Google Scholar]
- 39.Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001;7:291. doi: 10.1089/10763270152044152. [DOI] [PubMed] [Google Scholar]
- 40.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Xiao J, Zhou H, Zhao L, Sun Y, Guan S, Liu B, Kong L. The effect of hierarchical micro/nanosurface titanium implant on osseointegration in ovariectomized sheep. Osteoporos Int. 2011;22:1907. doi: 10.1007/s00198-010-1413-0. [DOI] [PubMed] [Google Scholar]
- 42.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27:5561. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 43.Benoit DS, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 44.Minagar S, Berndt CC, Wang J, Ivanova E, Wen C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.04.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Vogler EA, Bussian RW. Short-term cell attachment rates: A surface-sensitive test of cell-substrate compatibility. J Biomed Mater Res. 1987;21:1197. doi: 10.1002/jbm.820211004. [DOI] [PubMed] [Google Scholar]
- 46.Underwood PA, Bennett FA. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J Cell Sci. 1989;93:641. doi: 10.1242/jcs.93.4.641. [DOI] [PubMed] [Google Scholar]
- 47.Fabrizius-Homan DJ, Cooper SL. Competitive adsorption of vitronectin with albumin, fibrinogen, and fibronectin on polymeric biomaterials. J Biomed Mater Res. 1991;25:953. doi: 10.1002/jbm.820250804. [DOI] [PubMed] [Google Scholar]
- 48.Ranucci CS, Moghe PV. Substrate microtopography can enhance cell adhesive and migratory responsiveness to matrix ligand density. J Biomed Mater Res. 2001;54:149. doi: 10.1002/1097-4636(200102)54:2<149::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 49.Ugarova TP, Zamarron C, Veklich Y, Bowditch RD, Ginsberg MH, Weisel JW, Plow EF. Conformational transitions in the cell binding domain of fibronectin. Biochemistry. 1995;34:4457. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- 50.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF. TGF beta and BMP Bone. 1996 Jul;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 51.Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]