Abstract
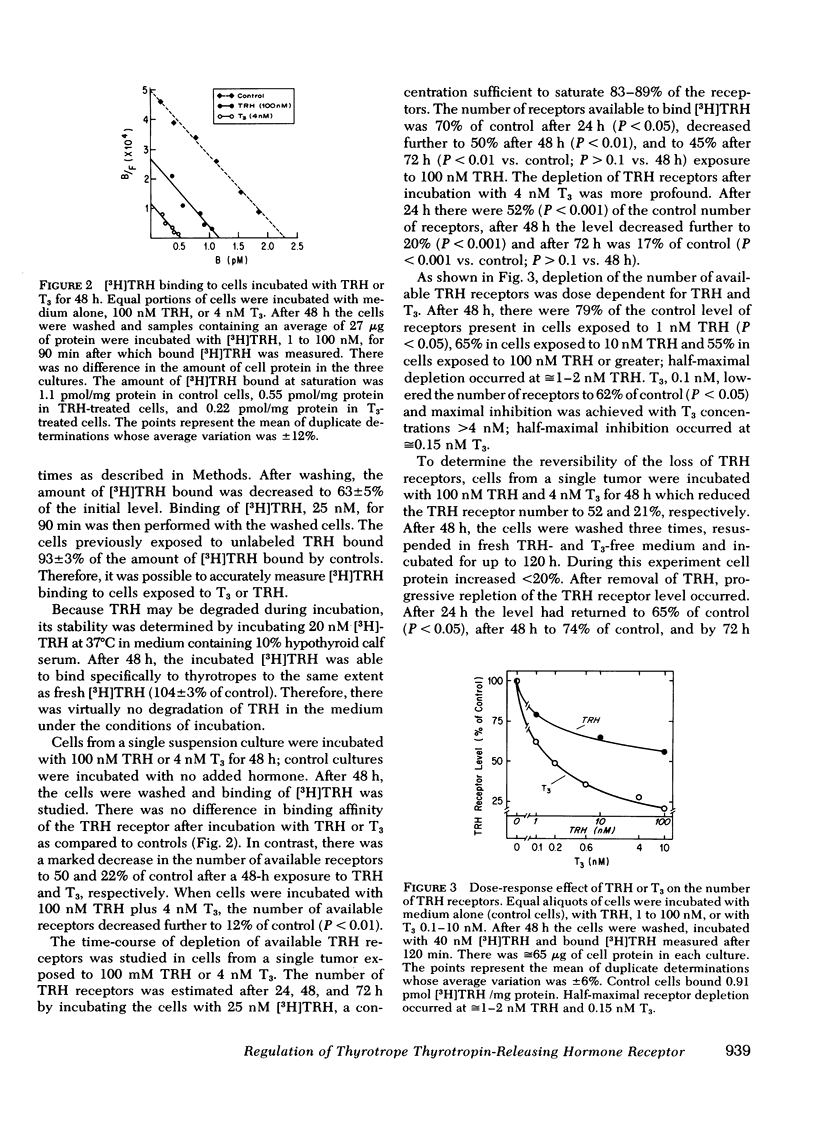
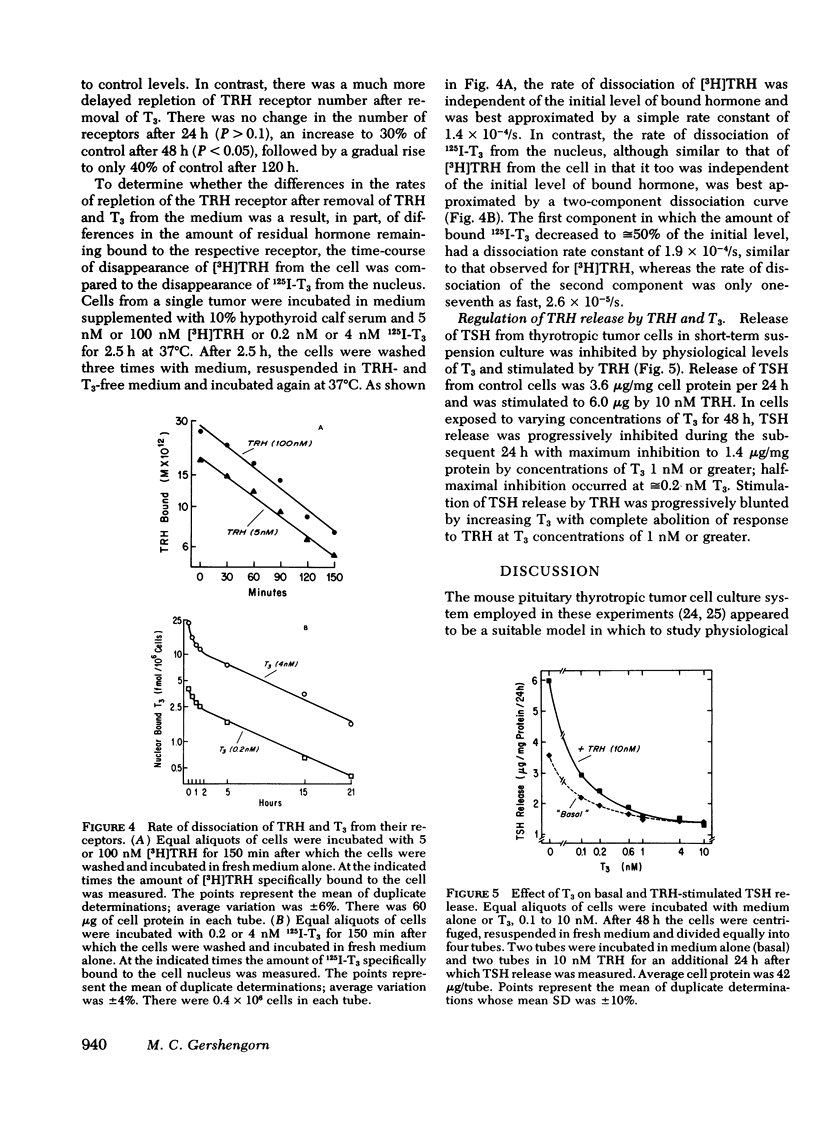
Receptors for thyrotropin-releasing hormone (TRH) are present on mouse pituitary thyrotropic tumor cells. Incubation of thyrotropes with 100 nM TRH or 4 nM L-triiodothyronine (T3) for 48 h decreased the number of TRH receptors to approximately equal to 50 and 20% of control, respectively. There was no effect on the equilibrium dissociation constant which was 3-5 nM. The depletion in the number of available TRH receptors was time- and dose-dependent. TRH, 100 nM, decreased the receptor number to 70% after 24 h, 50% after 48 h, and 45% of control after 72 h. T3, 4 nM, decreased the receptor number to 52% after 24 h, 20% after 48 h, and 17% of control after 72 h. After 48 h, half-maximal depletion occurred with 1-2 nM TRH and approximately equal to 0.15 nM T3. Incubation with 100 nM TRH and 4 nM T3 caused a significantly greater reduction in the receptor level than either hormone alone. The decrease in the receptor level was reversible within 72 h after removal of TRH, 100 nM, but was only partially reversed, from 20 to 40% of control, after removal of T3, 4 nM, after 120 h. By regulating the number of available TRH receptors on the thyrotrope. TRH and T3 interact to control thyrotropin release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M. R., Gershengorn M. C., Weintraub B. D. Excess production of free alpha subunits by mouse pituitary thyrotropic tumor cells in vitro. Endocrinology. 1978 Feb;102(2):499–508. doi: 10.1210/endo-102-2-499. [DOI] [PubMed] [Google Scholar]
- Bowers C. Y., Schally A. V., Reynolds G. A., Hawley W. D. Interactions of L-thyroxine or L-triiodothyronine and thyrotropin-releasing factor on the release and synthesis of thyrotropin from the anterior pituitary gland of mice. Endocrinology. 1967 Oct;81(4):741–747. doi: 10.1210/endo-81-4-741. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Eddy L. J., Hershman J. M., Taylor R. E., Jr, Barker S. B. Binding of thyrotropin releasing hormone by thyrotropin-secreting cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):140–146. doi: 10.1016/0006-291x(73)90900-5. [DOI] [PubMed] [Google Scholar]
- Eto S., Fleischer N. Regulation of thyrotropin (TSH) release and production in monolayer cultures of transplantable TSH-producing mouse tumors. Endocrinology. 1976 Jan;98(1):114–122. doi: 10.1210/endo-98-1-114. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Cohen M., Hoffstein S. T. Cellular heterogeneity in primary monolayer cultures of mouse pituitary thyrotropic tumors: separation of thyrotrophs. Endocrinology. 1978 Aug;103(2):648–651. doi: 10.1210/endo-103-2-648. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Regulation of thyrotropin production by mouse pituitary thyrotropic tumor cells in vitro by physiological levels of thyroid hormones. Endocrinology. 1978 Apr;102(4):1122–1128. doi: 10.1210/endo-102-4-1122. [DOI] [PubMed] [Google Scholar]
- Grant G., Vale W., Guillemin R. Characteristics of the pituitary binding sites for thyrotropin releasing factor. Endocrinology. 1973 Jun;92(6):1629–1633. doi: 10.1210/endo-92-6-1629. [DOI] [PubMed] [Google Scholar]
- Grant G., Vale W., Guillemin R. Interaction of thyrotropin releasing factor with membrane receptors of pituitary cells. Biochem Biophys Res Commun. 1972 Jan 14;46(1):28–34. doi: 10.1016/0006-291x(72)90625-0. [DOI] [PubMed] [Google Scholar]
- Gual C., Kastin A. J., Schally A. V. Clinical experience with hypothalamic releasing hormones. 1. Thyrotropin-releasing hormone. Recent Prog Horm Res. 1972;28:173–200. [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Receptors for thyrotropin-releasing hormone in prolactin producing rat pituitary cells in culture. J Biol Chem. 1973 Sep 10;248(17):6180–6186. [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry. 1975 Aug 26;14(17):3845–3851. doi: 10.1021/bi00688a017. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Woroch E. L., Tashjian A. H., Jr Receptor-binding affinities and biological activities of analogs of thyrotropin-releasing hormone in prolactin-producing pituitary cells in culture. J Biol Chem. 1974 May 25;249(10):3085–3090. [PubMed] [Google Scholar]
- Jacobs L. S., Snyder P. J., Wilber J. F., Utiger R. D., Daughaday W. H. Increased serum prolactin after administration of synthetic thyrotropin releasing hormone (TRH) in man. J Clin Endocrinol Metab. 1971 Dec;33(6):996–998. doi: 10.1210/jcem-33-6-996. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labrie F., Barden N., Poirier G., De Lean A. Binding of thyrotropin-releasing hormone to plasma membranes of bovine anterior pituitary gland (hormone receptor-adenylate cyclase-equilibrium constant-( 3 H)thyrotropin). Proc Natl Acad Sci U S A. 1972 Jan;69(1):283–287. doi: 10.1073/pnas.69.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léan A. D., Ferland L., Drouin J., Kelly P. A., Labrie F. Modulation of pituitary thyrotropin releasing hormone receptor levels by estrogens and thyroid hormones. Endocrinology. 1977 Jun;100(6):1496–1504. doi: 10.1210/endo-100-6-1496. [DOI] [PubMed] [Google Scholar]
- May P. B., Donabedian R. K. Thyrotropin releasing hormone (TRH) mediated thyroid-stimulating hormone (TSH) release from human anterior pituitary tissue in vitro. J Clin Endocrinol Metab. 1973 Mar;36(3):605–607. doi: 10.1210/jcem-36-3-605. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone M. H., Hinkle P. M. Regulation of pituitary receptors for thyrotropin-releasing hormone by thyroid hormones. J Biol Chem. 1978 Jul 25;253(14):5168–5173. [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Induction of a lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2407–2410. doi: 10.1073/pnas.71.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman L., Mitsuma T., Hollander C. S. Modulation of pituitary responsiveness to thyrotropin-releasing hormone by triiodothyronine. J Clin Invest. 1973 Jan;52(1):205–209. doi: 10.1172/JCI107166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P. J., Utiger R. D. Inhibition of thyrotropin response to thyrotropin-releasing hormone by small quantities of thyroid hormones. J Clin Invest. 1972 Aug;51(8):2077–2084. doi: 10.1172/JCI107014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Barowsky N. J., Jensen D. K. Thyrotropin releasing hormone: direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem Biophys Res Commun. 1971 May 7;43(3):516–523. doi: 10.1016/0006-291x(71)90644-9. [DOI] [PubMed] [Google Scholar]
- Wilber J. F. Stimulation of 14-C-glucosamine and 14-C-alanine incorporation into thyrotropin by synthetic thyrotropin-releasing hormone. Endocrinology. 1971 Sep;89(3):873–877. doi: 10.1210/endo-89-3-873. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. In vitro studies on mechanism of action of thyrotropin releasing factor. Proc Soc Exp Biol Med. 1968 Feb;127(2):488–490. doi: 10.3181/00379727-127-32722. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


