Abstract
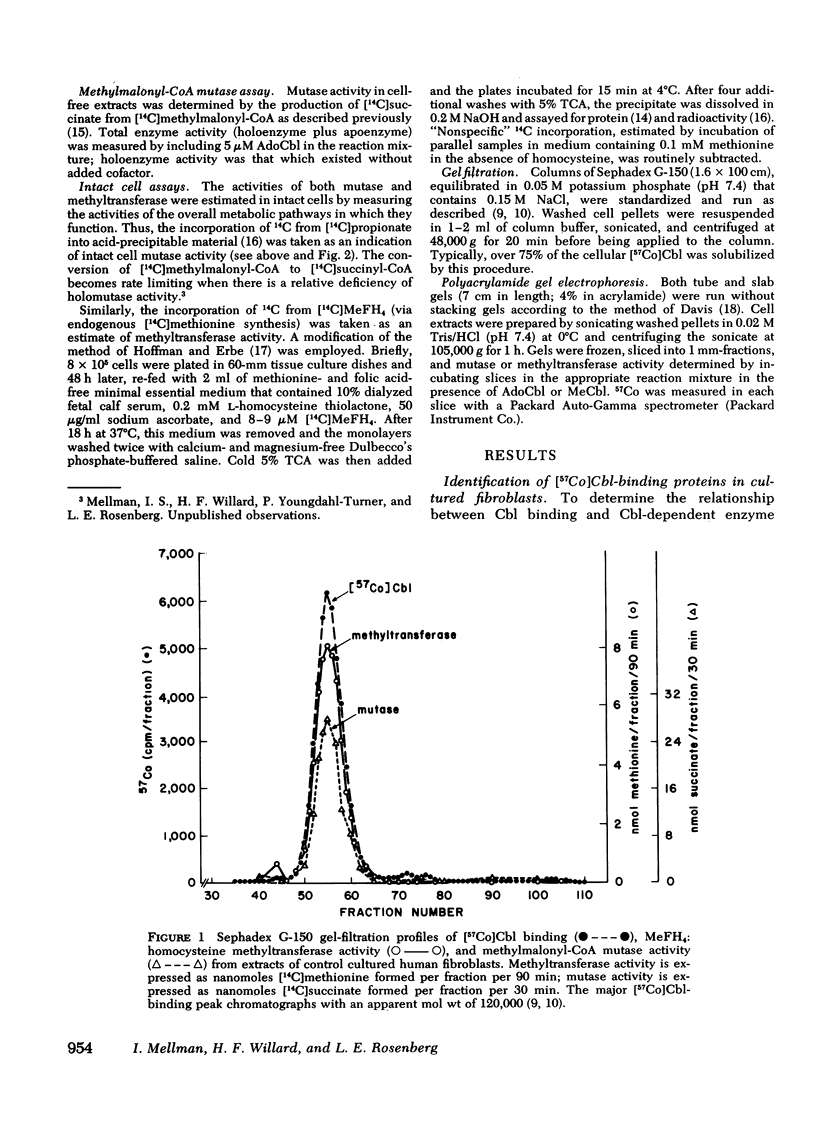
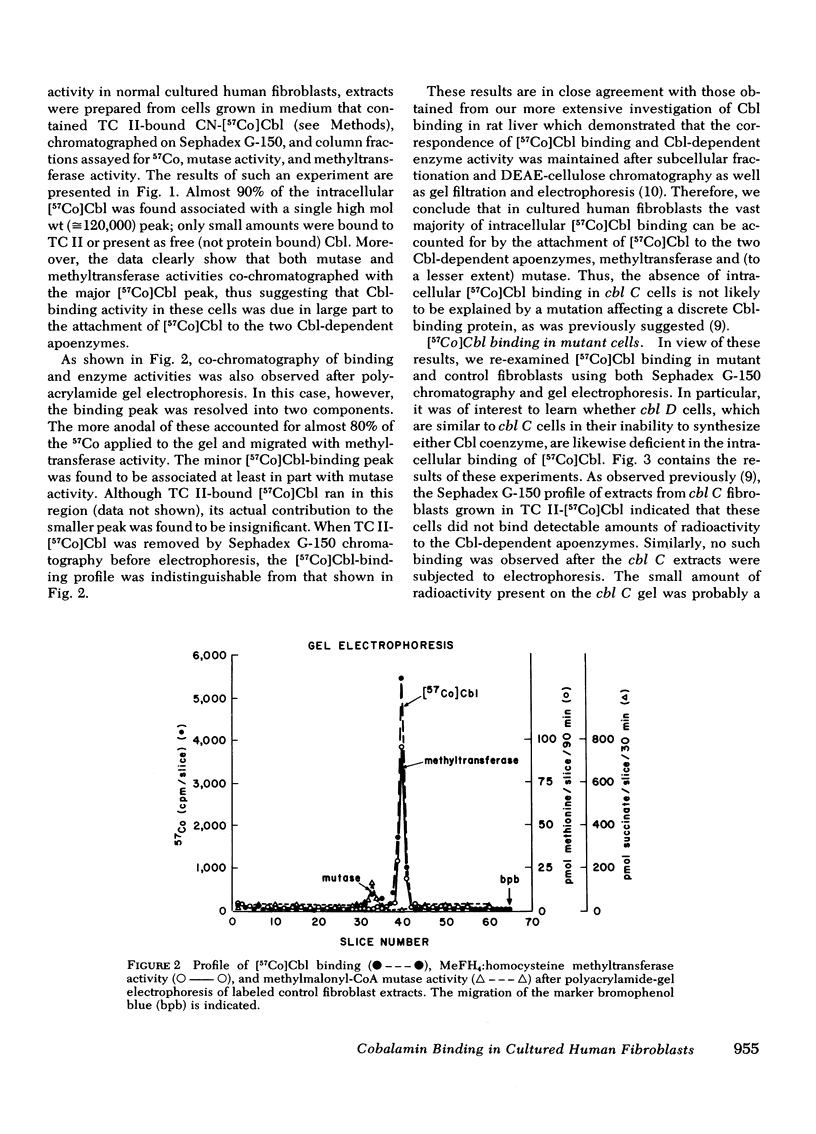
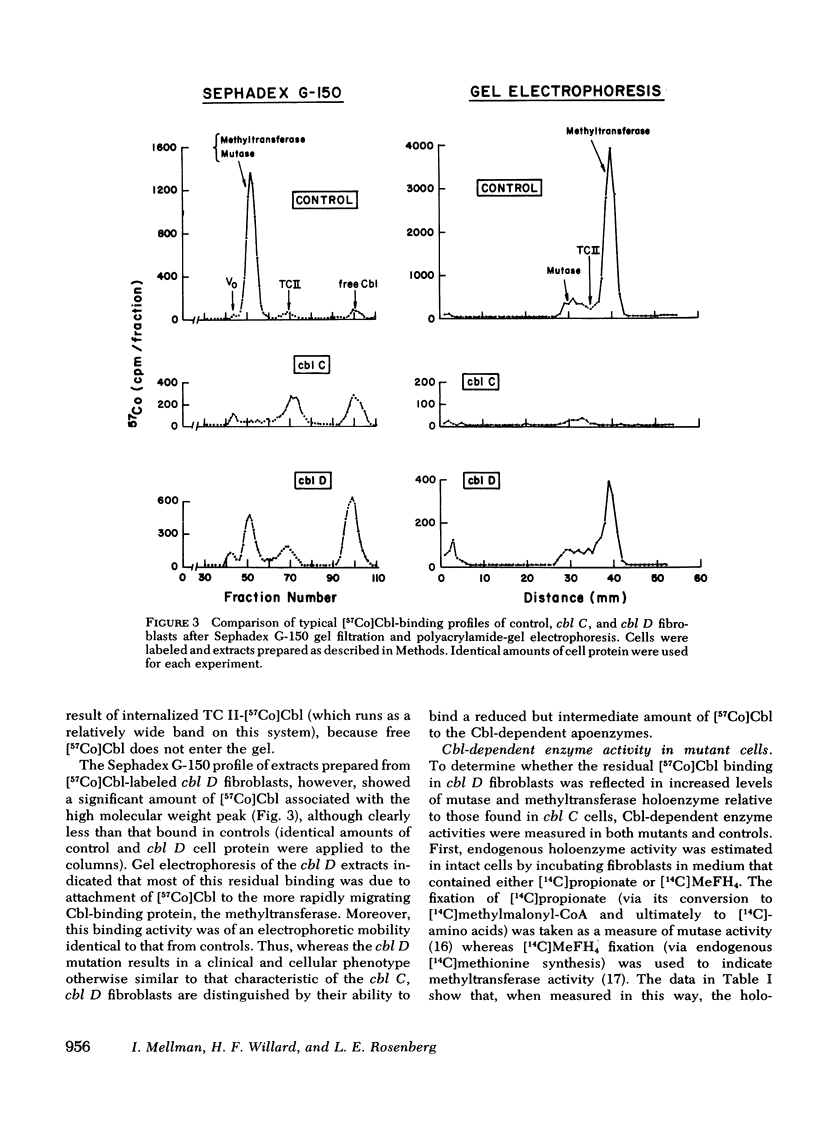
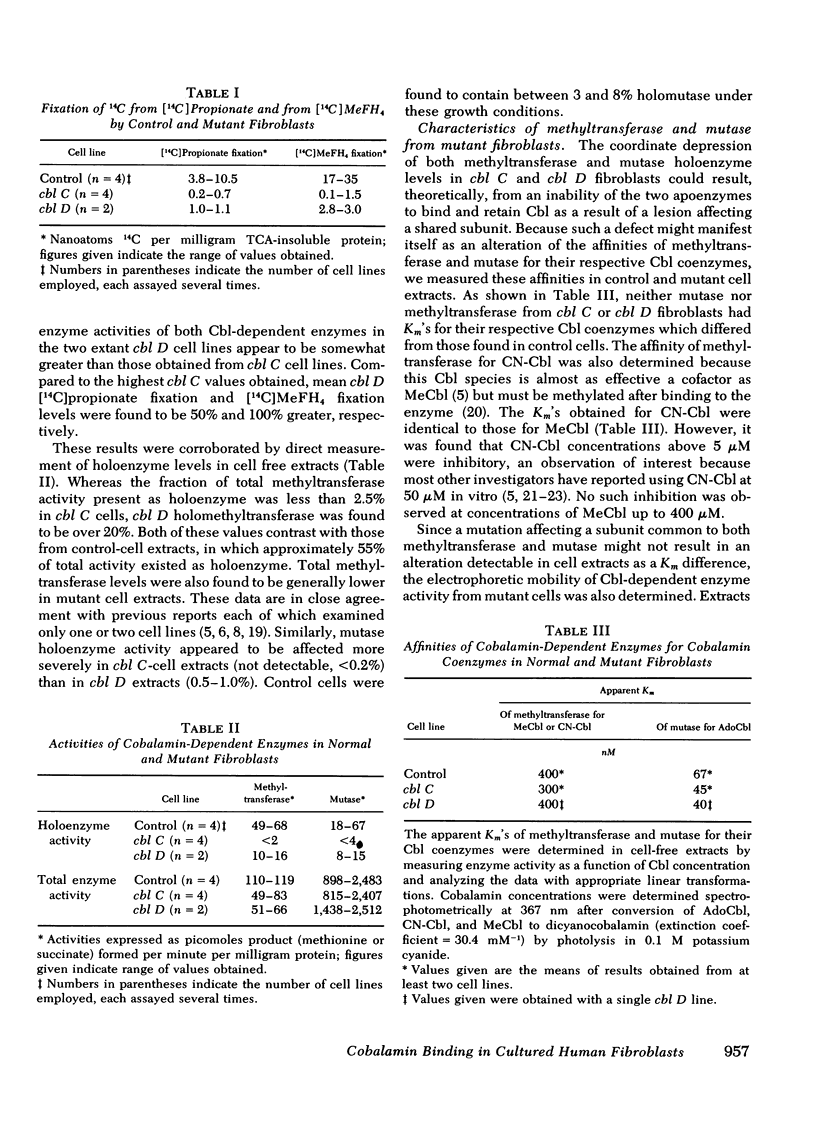
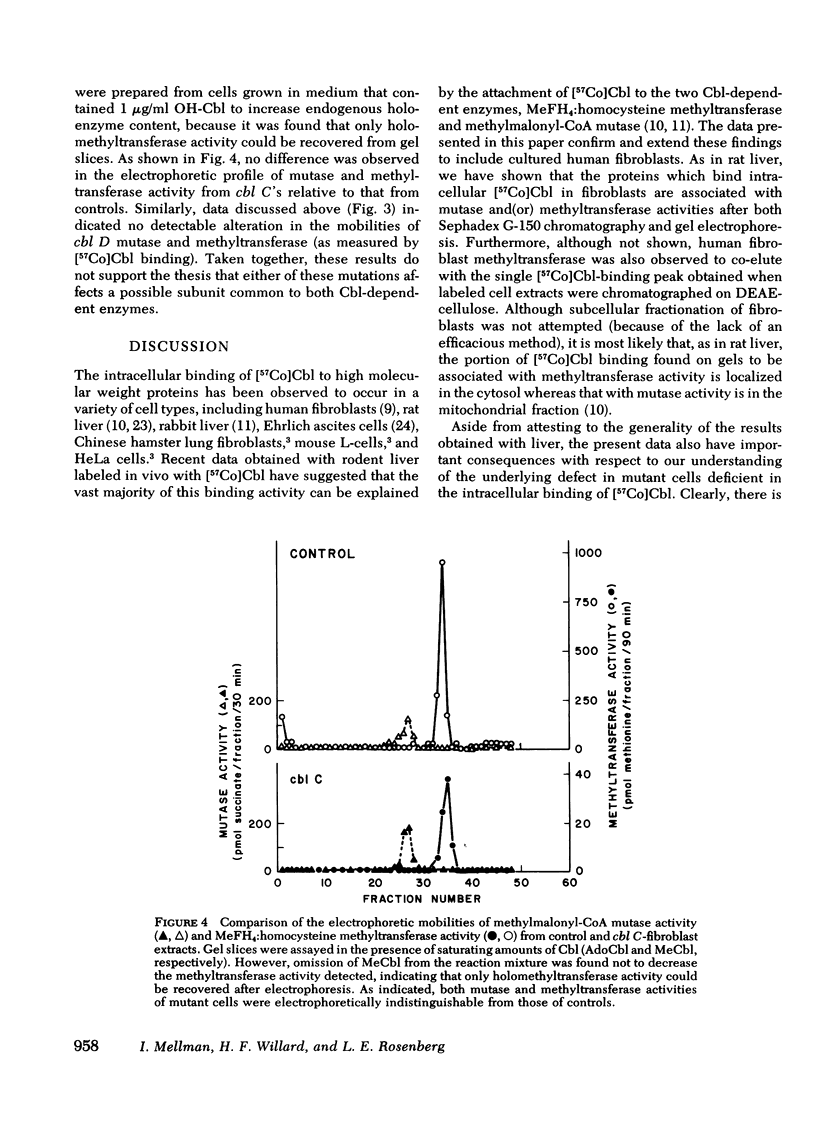
We have studied the intracellular binding of radioactive cobalamin by normal cultured human fibroblasts grown in medium containing [57Co]-cobalamin. We have also assessed the significance of defects in this binding activity exhibited by two classes of human mutants (cbl C and cbl D) each characterized by pleiotropic deficiencies in the accumulation and retention of cobalamin, in the synthesis of cobalamin coenzymes, and accordingly, in the holoenzyme activities of both cobalamin-dependent enzymes, 5-methyltetrahydrofolate:homocysteine methyltransferase and methylmalonyl-CoA mutase. Based on the coincidence of [57Co]cobalamin binding and cobalamin-dependent enzyme activities after Sephadex G-150 chromatography and polyacrylamide gel electrophoresis, we conclude that, as in rat liver, the intracellular binding of labeled cobalamin by normal fibroblasts reflects the attachment of the vitamin to the cobalamin-dependent methyltransferase and mutase. Whereas cbl C cells are completely deficient in the binding of [57Co]cobalamin to either enzyme, fibroblasts which bear the phenotypically similar but genetically distinct cbl D mutation retain some binding activity, and accordingly, have higher holomethyltransferase and holomutase activities than do cbl C cells. The defect in [57Co]-cobalamin binding exhibited by both cbl C and cbl D fibroblasts is almost certainly not a result of mutations which affect the methyltransferase or mutase apoenzymes, since the electrophoretic mobilities and the affinities of these enzymes for their respective cobalamin coenzymes are indistinguishable from those in control cell extracts. These results suggest that both the cbl C and cbl D mutations affect some enzymatic step(s) which converts newly taken up cobalamin to a form capable of being bound by the two cobalamin-dependent enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe H., Clark B. R., Chu F., Hardy D. N., Halpern B. C., Halpern R. M., Smith R. A. N5-methyltetrahydrofolate: homocysteine methyltransferase activity in extracts from normal, malignant and embryonic tissue culture cells. Biochem Biophys Res Commun. 1974 Mar 25;57(2):417–425. doi: 10.1016/0006-291x(74)90947-4. [DOI] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke G. T., Mangum J. H., Brodie J. D. Methylcobalamin as an intermediate in mammalian methionine biosynthesis. Biochemistry. 1970 Oct 27;9(22):4297–4302. doi: 10.1021/bi00824a009. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Urlaub G. Mutant alleles for hypoxanthine phosphoriboxyltransferase: codominant expression, complementation, and segregation in hybrid Chinese hamster cells. Somatic Cell Genet. 1976 Sep;2(5):453–467. doi: 10.1007/BF01542725. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dillon M. J., England J. M., Gompertz D., Goodey P. A., Grant D. B., Hussein H. A., Linnell J. C., Matthews D. M., Mudd S. H., Newns G. H. Mental retardation, megaloblastic anaemia, methylmalonic aciduria and abnormal homocysteine metabolism due to an error in vitamin B12 metabolism. Clin Sci Mol Med. 1974 Jul;47(1):43–61. doi: 10.1042/cs0470043. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Ambani L. M., Rosenberg L. E. Uptake of hydroxocobalamin by rat liver mitochondria. Binding to a mitochondrial protein. J Biol Chem. 1976 Nov 10;251(21):6616–6623. [PubMed] [Google Scholar]
- Fenton W. A., Rosenberg L. E. Genetic and biochemical analysis of human cobalamin mutants in cell culture. Annu Rev Genet. 1978;12:223–248. doi: 10.1146/annurev.ge.12.120178.001255. [DOI] [PubMed] [Google Scholar]
- Frenkel E. P., Kitchens R. L. Intracellular localization of hepatic propionyl-CoA carboxylase and methylmalonyl-CoA mutase in humans and normal and vitamin B12 deficient rats. Br J Haematol. 1975 Dec;31(4):501–513. doi: 10.1111/j.1365-2141.1975.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., Moe P. G., Hammond K. B., Mudd S. H., Uhlendorf B. W. Homocystinuria with methylmalonic aciduria: two cases in a sibship. Biochem Med. 1970 Dec;4(5):500–515. doi: 10.1016/0006-2944(70)90080-3. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Mahoney M. J., Ruddle F. H., Rosenberg L. E. Genetic complementation in heterokaryons of human fibroblasts defective in cobalamin metabolism. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3181–3185. doi: 10.1073/pnas.72.8.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. M., Erbe R. W. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci U S A. 1976 May;73(5):1523–1527. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamely D., Littlefield J. W., Erbe R. W. Regulation of 5-methyltetrahydrofolate: homocysteine methyltransferase activity by methionine, vitamin B12, and folate in cultured baby hamster kidney cells. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2585–2589. doi: 10.1073/pnas.70.9.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Recognition of two intracellular cobalamin binding proteins and their identification as methylmalonyl-CoA mutase and methionine synthetase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):921–925. doi: 10.1073/pnas.74.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnell J. C., Matthews D. M., Mudd S. H., Uhlendorf B. W., Wise I. J. Cobalamins in fibroblasts cultured from normal control subjects and patients with methylmalonic aciduria. Pediatr Res. 1976 Mar;10(3):179–183. doi: 10.1203/00006450-197603000-00007. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Hart A. C., Steen V. D., Rosenberg L. E. Methylmalonicacidemia: biochemical heterogeneity in defects of 5'-deoxyadenosylcobalamin synthesis. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2799–2803. doi: 10.1073/pnas.72.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E., Mudd S. H., Uhlendorf B. W. Defective metabolism of vitamin B 12 in fibroblasts from children with methylmalonicaciduria. Biochem Biophys Res Commun. 1971 Jul 16;44(2):375–381. doi: 10.1016/0006-291x(71)90610-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Youngdahl-Turner P., Willard H. F., Rosenberg L. E. Intracellular binding of radioactive hydroxocobalamin to cobalamin-dependent apoenzymes in rat liver. Proc Natl Acad Sci U S A. 1977 Mar;74(3):916–920. doi: 10.1073/pnas.74.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Levy H., Morrow G., 3rd Deranged B 12 metabolism: effects on sulfur amino acid metabolism. Biochem Med. 1970 Nov;4(3):193–214. doi: 10.1016/0006-2944(70)90049-9. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Uhlendorf B. W., Hinds K. R. Deranged B 12 metabolism: studies of fibroblasts grown in tissue culture. Biochem Med. 1970 Nov;4(3):215–239. doi: 10.1016/0006-2944(70)90050-5. [DOI] [PubMed] [Google Scholar]
- Peirce K., Abe T., Cooper B. A. Incorporation and metabolic conversion of cyanocobalamin by Ehrlich ascites carcinoma cells in vitro and in vivo. Biochim Biophys Acta. 1975 Feb 13;381(2):348–358. doi: 10.1016/0304-4165(75)90240-8. [DOI] [PubMed] [Google Scholar]
- Pletsch Q. A., Coffey J. W. Properties of the proteins that bind vitamin B 12 in subcellular fractions of rat liver. Arch Biochem Biophys. 1972 Jul;151(1):157–167. doi: 10.1016/0003-9861(72)90484-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Patel L., Lilljeqvist A. C. Absence of an intracellular cobalamin-binding protein in cultured fibroblasts from patients with defective synthesis of 5'-deoxyadenosylcobalamin and methylcobalamin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4617–4621. doi: 10.1073/pnas.72.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- Willard H. F., Ambani L. M., Hart A. C., Mahoney M. J., Rosenberg L. E. Rapid prenatal and postnatal detection of inborn errors of propionate, methylmalonate, and cobalamin metabolism: a sensitive assay using cultured cells. Hum Genet. 1976 Dec 15;34(3):277–283. doi: 10.1007/BF00295291. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Mellman I. S., Rosenberg L. E. Genetic complementation among inherited deficiencies of methylmalonyl-CoA mutase activity: evidence for a new class of human cobalamin mutant. Am J Hum Genet. 1978 Jan;30(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inherited deficiencies of human methylmalonyl CaA mutase activity: reduced affinity of mutant apoenzyme for adenosylcobalamin. Biochem Biophys Res Commun. 1977 Oct 10;78(3):927–934. doi: 10.1016/0006-291x(77)90511-3. [DOI] [PubMed] [Google Scholar]
- Youngdahl-Turner P., Rosenberg L. E., Allen R. H. Binding and uptake of transcobalamin II by human fibroblasts. J Clin Invest. 1978 Jan;61(1):133–141. doi: 10.1172/JCI108911. [DOI] [PMC free article] [PubMed] [Google Scholar]


