Abstract
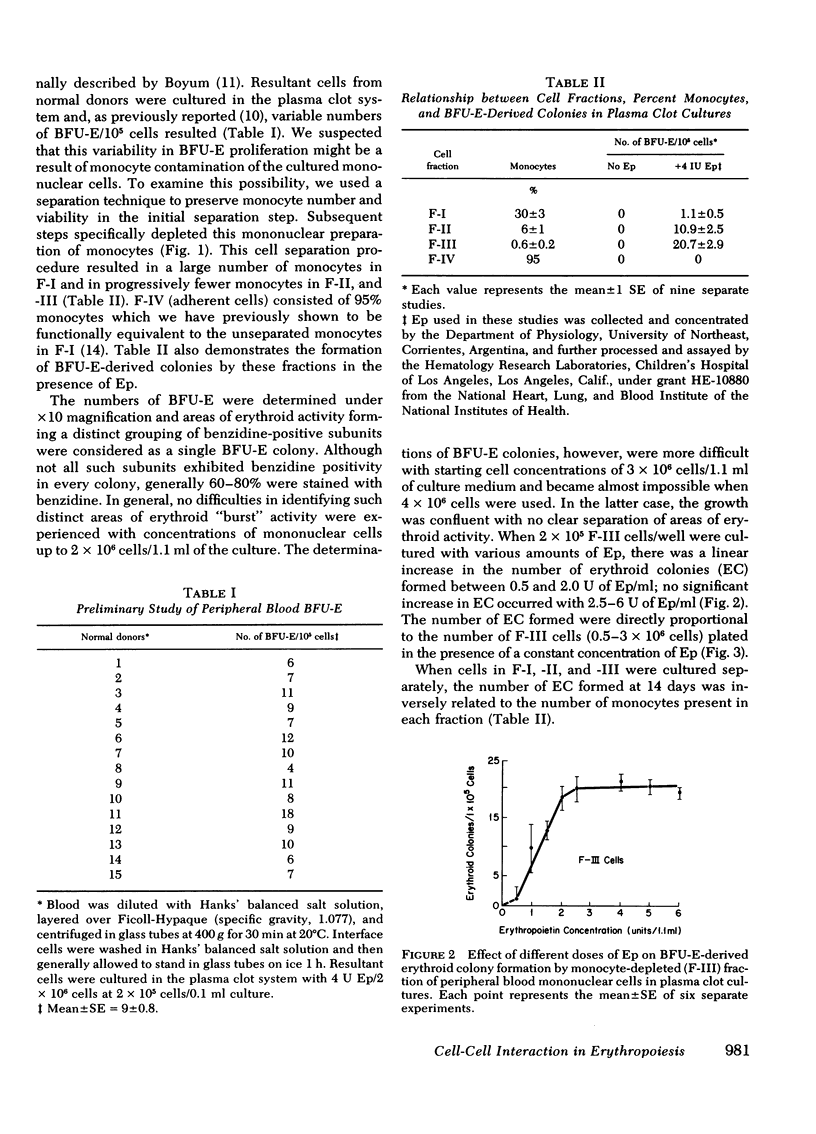
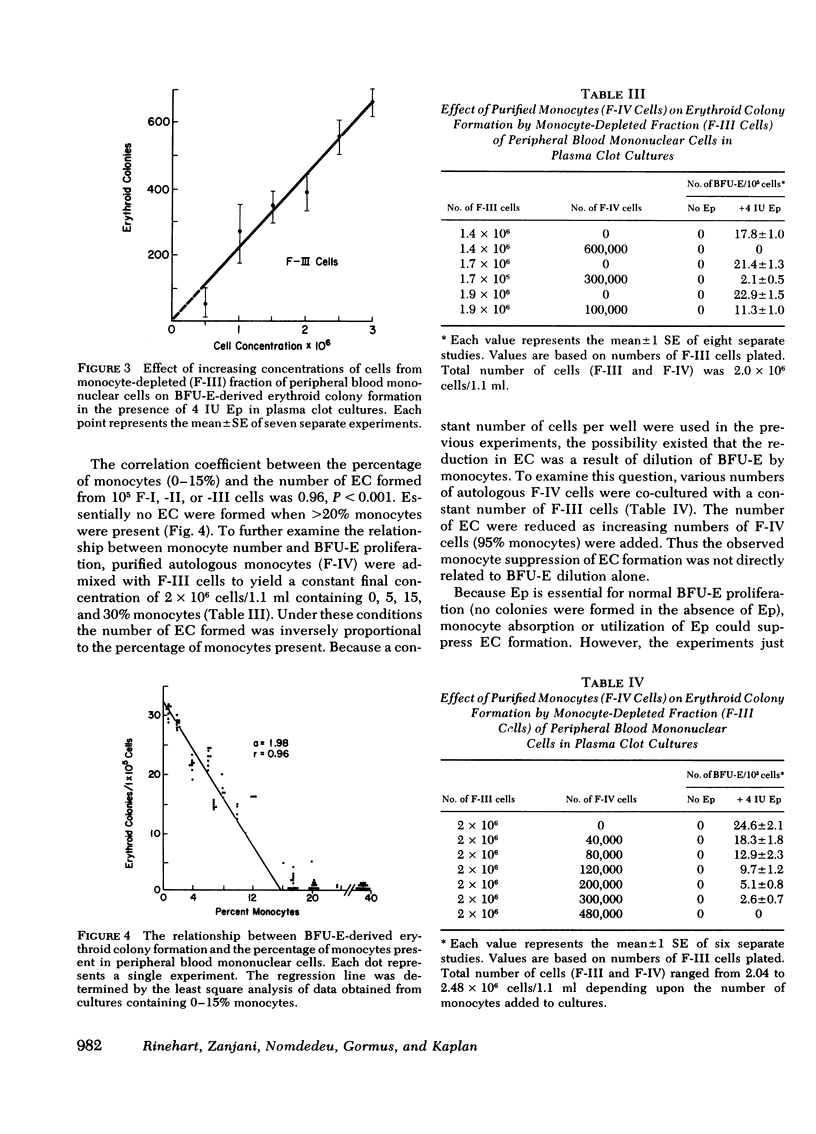
Erythroid burst forming units (BFU-E) are proliferative cells present in peripheral blood and bone marrow which may be precursors of the erythroid colony forming cell found in the bone marrow. To examine the possible role of monocyte-macrophages in the modulation of erythropoiesis, the effect of monocytes on peripheral blood BFU-E proliferation in response to erythropoietin was investigated in the plasma clot culture system. Peripheral blood mononuclear cells from normal human donors were separated into four fractions. Fraction-I cells were obtained from the interface of Ficoll-Hypaque gradients (20-30% monocytes; 60-80% lymphocytes); fraction-II cells were fraction-I cells that were nonadherent to plastic (2-10% monocytes; 90-98% lymphocytes); fraction-III cells were obtained by incubation of fraction-II cells with carbonyl iron followed by Ficoll-Hypaque centrifugation (>99% lymphocytes); and fraction-IV cells represented the adherent population of fraction-II cells released from the plastic by lidocaine (>95% monocytes). When cells from these fractions were cultured in the presence of erythropoietin, the number of BFU-E-derived colonies was inversely proportional to the number of monocytes present (r = −0.96, P < 0.001). The suppressive effect of monocytes on BFU-E proliferation was confirmed by admixing autologous purified monocytes (fraction-IV cells) with fraction-III cells. Monocyte concentrations of ≥20% completely suppressed BFU-E activity. Reduction in the number of plated BFU-E by monocyte dilution could not account for these findings: a 15% reduction in the number of fraction-III cells plated resulted in only a 15% reduction in colony formation. These results indicate that monocyte-macrophages may play a significant role in the regulation of erythropoiesis and be involved in the pathogenesis of the hypoproliferative anemias associated with infection and certain neoplasia in which increased monocyte activity and monopoiesis also occur.
Full text
PDF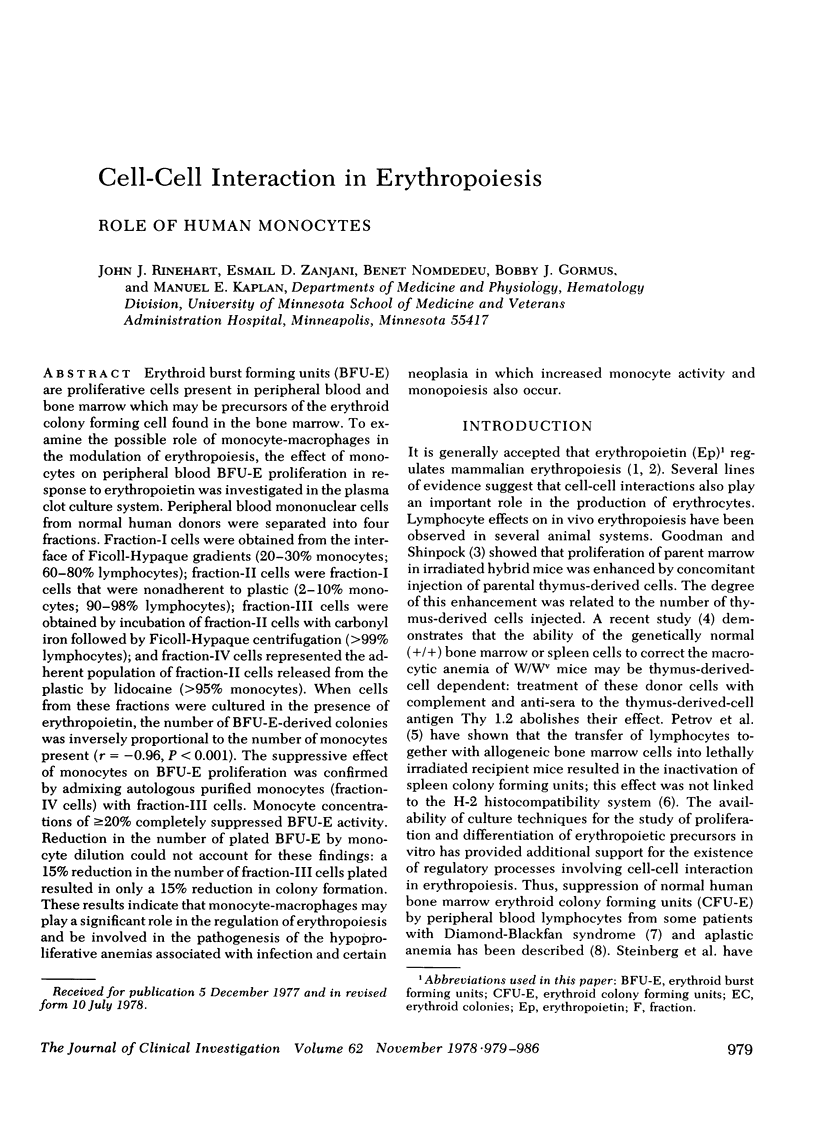





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye M. T. Erythroid colony formation in cultures of human marrow: effect of leukocyte conditioned medium. J Cell Physiol. 1977 Apr;91(1):69–77. doi: 10.1002/jcp.1040910108. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CHARNEY E., MILLER G. RETICULOCYTOPENIA IN SICKLE CELL DISEASE. APLASTIC EPISODES IN THE COURSE OF SICKLE CELL DISEASE IN CHILDREN. Am J Dis Child. 1964 May;107:450–455. doi: 10.1001/archpedi.1964.02080060452004. [DOI] [PubMed] [Google Scholar]
- CUDKOWICZ G., STIMPFLING J. H. DEFICIENT GROWTH OF C57BL MARROW CELLS TRANSPLANTED IN F1 HYBRID MICE. ASSOCIATION WITH THE HISTOCOMPATIBILITY-2 LOCUS. Immunology. 1964 May;7:291–306. [PMC free article] [PubMed] [Google Scholar]
- Camiscoli J. F., Weintraub A. H., Gordon A. S. Comparative assay of erythropoietin standards. Ann N Y Acad Sci. 1968 Mar 29;149(1):40–45. doi: 10.1111/j.1749-6632.1968.tb15133.x. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1105–1109. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein K., Preisler H. D., Lutton J. D., Zanjani E. D. Erythroid colony formation in vitro by dimethylsulfoxide-treated erythroleukemic cells. Blood. 1974 Dec;44(6):831–836. [PubMed] [Google Scholar]
- Goodman J. W., Shinpock S. G. Influence of thymus cells on erythropoiesis of parental marrow in irradiated hybrid mice. Proc Soc Exp Biol Med. 1968 Nov;129(2):417–422. doi: 10.3181/00379727-129-33334. [DOI] [PubMed] [Google Scholar]
- Gordon A. S. Erythropoietin. Vitam Horm. 1973;31:105–174. doi: 10.1016/s0083-6729(08)60997-8. [DOI] [PubMed] [Google Scholar]
- Haurani F. I., Meyer A. Iron and the reticuloendothelial system. Adv Exp Med Biol. 1976;73(PT-A):171–187. doi: 10.1007/978-1-4684-3297-8_16. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Lutton J. D., Zalusky R., Wasserman L. R. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. 1977 Jan 6;296(1):10–13. doi: 10.1056/NEJM197701062960103. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Vila J., Zalusky R., Lutton J. D., Wasserman L. R. Diamond-Blackfan syndrome: lymphocyte-mediated suppression of erythropoiesis. Science. 1976 Sep 3;193(4256):899–900. doi: 10.1126/science.986086. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Cudkowicz G. Abrogation of resistance to bone marrow grafts by silica particles. Prevention of the silica effect by the macrophage stabilizer poly-2-vinylpyridine N-oxide. J Immunol. 1974 Sep;113(3):798–803. [PubMed] [Google Scholar]
- Meuret G., Hoffmann G. Monocyte kinetic studies in normal and disease states. Br J Haematol. 1973 Mar;24(3):275–285. doi: 10.1111/j.1365-2141.1973.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Moore M. A. Regulatory role of macrophages in hemopoiesis. J Reticuloendothel Soc. 1976 Jul;20(1):89–91. [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov R. V., Khaitov R. M., Aleinikova N. V., Gulak L. V. Factors controlling stem cell recirculation. III. Effect of the thymus on the migration and differentiation of hemopoietic stem cells. Blood. 1977 Jun;49(6):865–872. [PubMed] [Google Scholar]
- Petrov R. V., Seslavina L. S., Pantelejev E. I., Yegorova O. S. Inactivation of stem cells by lymphocytes nonlinked to the H-2 histocompatibility system. Transplant Proc. 1977 Mar;9(1):555–557. [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Calderon J., Kiely J. M., Stadecker M. J. Regulation of immunity and inflammation by mediators from macrophages. Am J Pathol. 1976 Nov;85(2):465–478. [PMC free article] [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Sharkie S., Ahmed A., Sell K. W., Santos G. W. Theta-sensitive cell and erythropoiesis: identification of a defect in W/Wv anemic mice. Science. 1977 Apr 15;196(4287):313–315. doi: 10.1126/science.322288. [DOI] [PubMed] [Google Scholar]
- Zanjani E. D., Lutton J. D., Hoffman R., Wasserman L. R. Erythroid colony formation by polycythemia vera bone marrow in vitro. Dependence on erythropoietin. J Clin Invest. 1977 May;59(5):841–848. doi: 10.1172/JCI108706. [DOI] [PMC free article] [PubMed] [Google Scholar]


