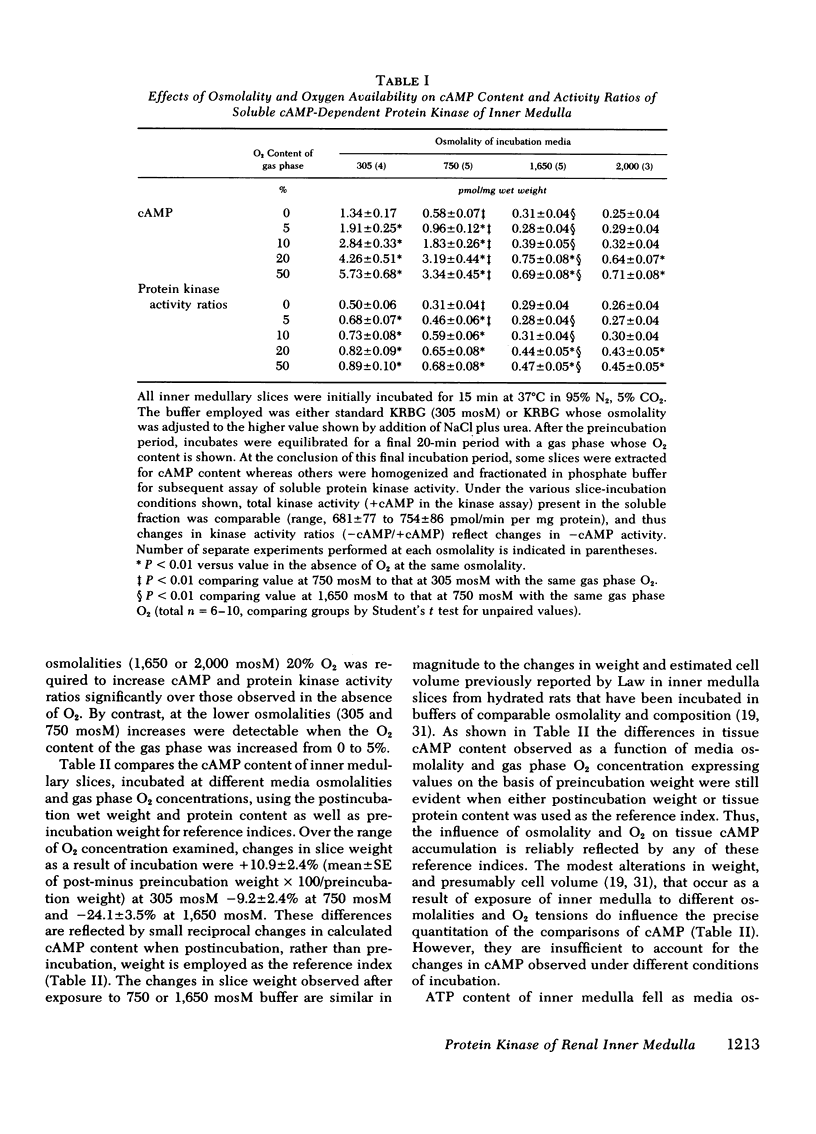
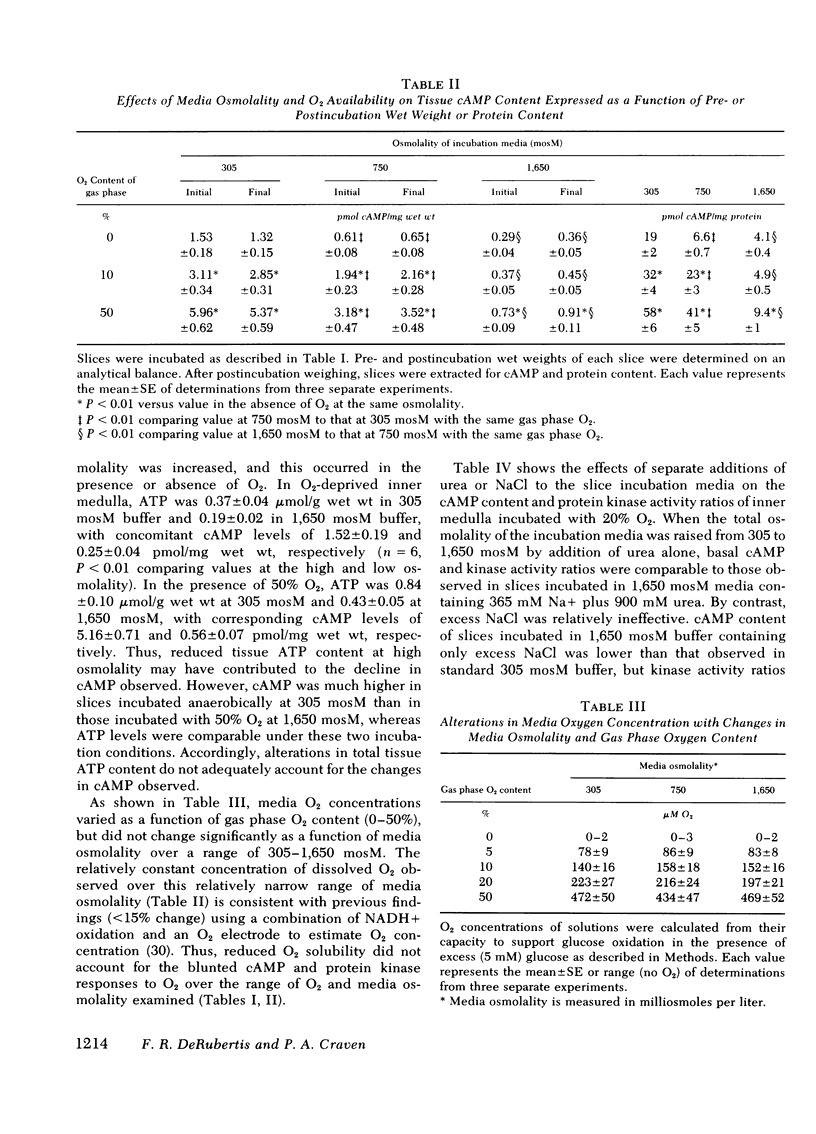
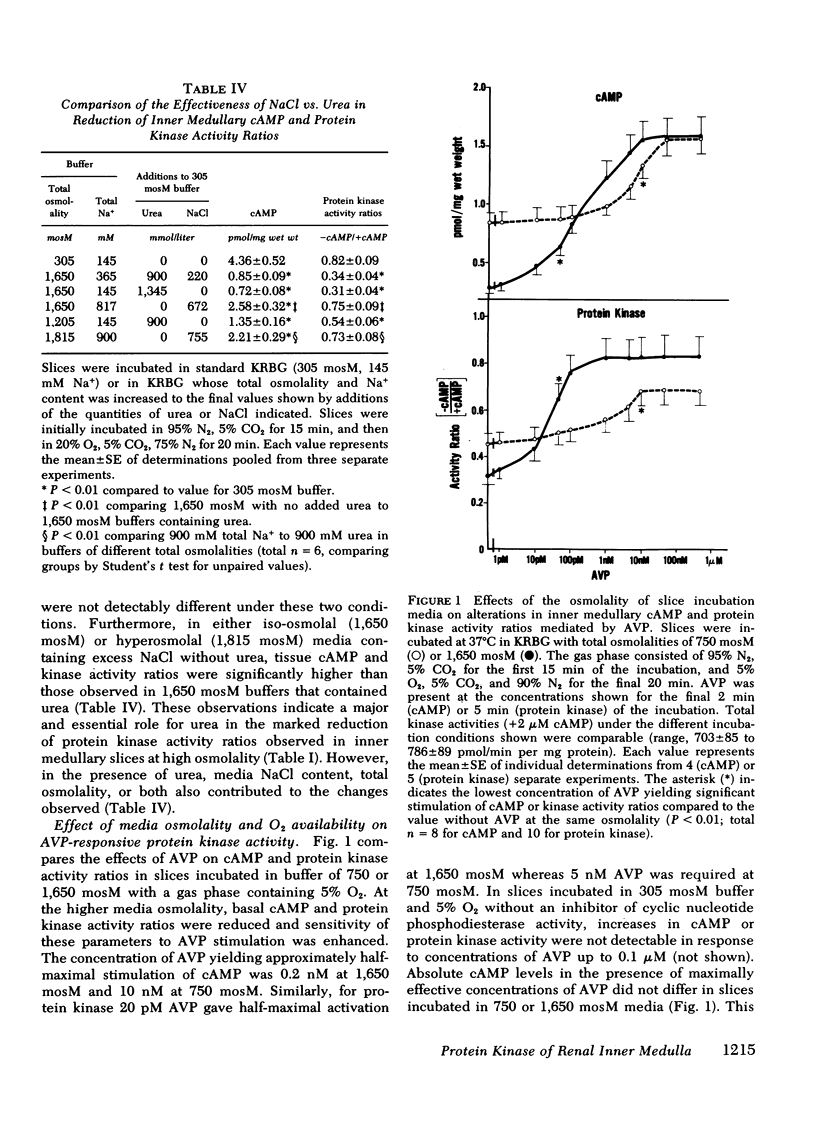
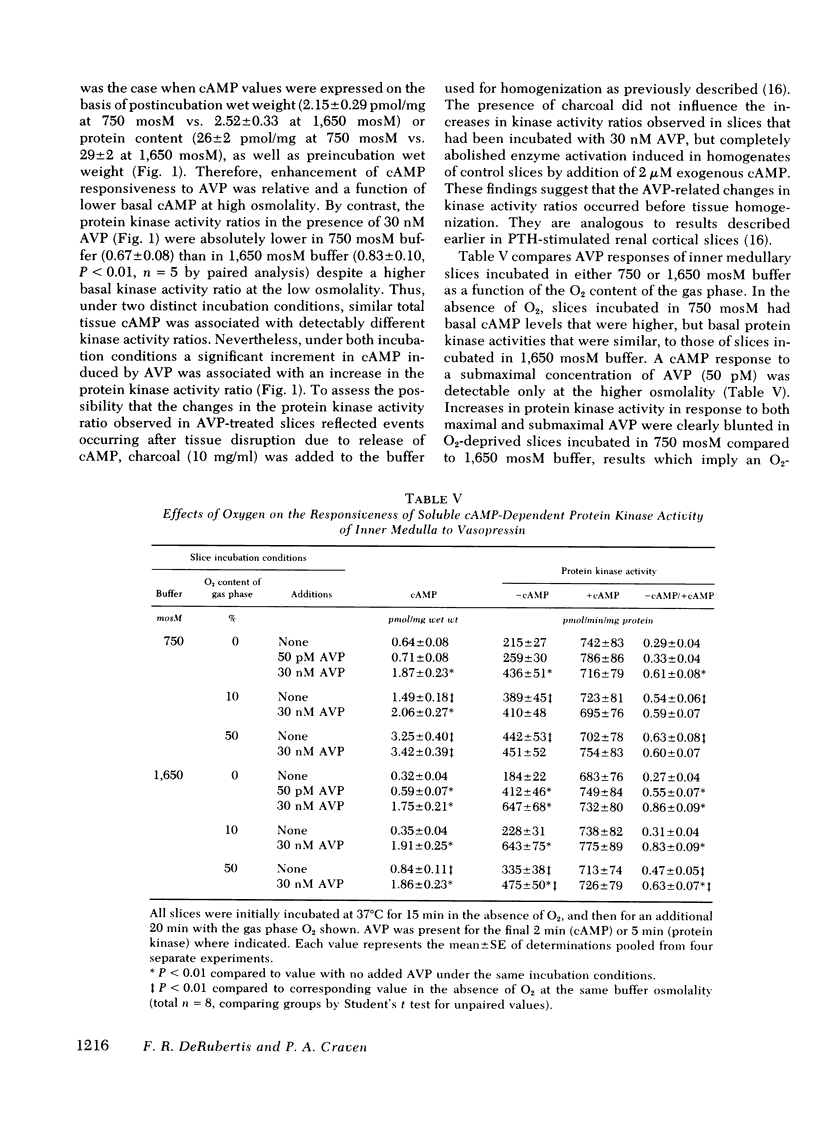
Abstract
The renal inner medulla is ordinarily exposed to osmolalities that are much higher and to O2 tensions that are lower than those in other tissues. The effects of media osmolality and O2 availability on basal and arginine vasopressin(AVP)-responsive soluble cyclic (c)AMP-dependent protein kinase activity were examined in slices of rat inner medulla. Increasing total media osmolality from 305 to 750 or 1,650 mosM by addition of urea plas NaCl to standard Krebs-Ringer bicarbonate buffer significantly reduced basal cAMP content and protein kinase activity ratios. This occurred in the presence or absence of O2. Incubation of slices in high osmolality buffer also blunted increases in inner medullary slice cAMP and protein kinase activity ratios induced by O2. These changes reflected predominantly an action of the urea rather than the NaCl content of high osmolality buffers. In contrast to effects on basal activity, high media osmolality significantly enhanced activation of inner medullary protein kinase by AVP. Conversely, increases in media O2 content suppressed AVP stimulation of enzyme activity. This inhibitory effect of O2 was best expressed at low osmolality. Naproxen and ibuprofen, inhibitors of prostaglandin biosynthesis, reduced basal kinase activity ratios and increased AVP responsiveness in the presence, but not in the absence, of O2. Exogenous prostaglandins (PG) modestly increased (PGE2 and PGE1) or did not change (PGF2alpha) cAMP and protein kinase activity ratios in O2-deprived inner medullary slices. Protein kinase activation by PGE2 was not observed in oxygenated inner medulla with high basal activity ratios. The stimulatory effects of PGE2 and PGE1 on protein kinase activity observed in O2-deprived slices were additive with those of submaximal or maximal AVP. PGE2, PGE1, and PGF2alpha all failed to suppress AVP activation of protein kinase. Thus, enhanced endogenous PGE production may contribute to the higher basal protein kinase activity ratios induced by O2. However, the results do not support a role for PGE2, PGE1, or PGF2alpha in O2-mediated inhibition of AVP responsiveness. The present data indicate that both solute content and O2 availability can alter the expression of AVP action on cAMP-dependent protein kinase activity in inner medulla. AVP activation of protein kinase is best expressed when osmolality is high and O2 availability is low, conditions that pertain in inner medulla during hydropenia.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APERIA A. C., LIEBOW A. A. IMPLICATIONS OF URINE PO2 FOR RENAL MEDULLARY BLOOD FLOW. Am J Physiol. 1964 Mar;206:499–504. doi: 10.1152/ajplegacy.1964.206.3.499. [DOI] [PubMed] [Google Scholar]
- APPELBOOM J. W., BRODSKY W. A., SCOTT W. N. EFFECT OF OSMOTIC DIURESIS ON INTRARENAL SOLUTES IN DIABETES INSIPIDUS AND HYDROPENIA. Am J Physiol. 1965 Jan;208:38–45. doi: 10.1152/ajplegacy.1965.208.1.38. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J. C., Hai M. A., Thomas S. The time course of changes in renal tissue composition during mannitol diuresis in the rat. J Physiol. 1968 Jul;197(2):411–428. doi: 10.1113/jphysiol.1968.sp008567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J. C., Hai M. A., Thomas S. The time course of changes in renal tissue composition duruig water diuresis in the rat. J Physiol. 1968 Jul;197(2):429–443. doi: 10.1113/jphysiol.1968.sp008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C. Studies on receptor-mediated activation of adenylyl cyclases. I. Preparation and description of general properties of an adenylyl cyclase system in beef renal medullary membranes sensitive to neurohypophyseal hormones. J Biol Chem. 1974 Dec 25;249(24):7848–7856. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- DE WARDENER H. E., HERXHEIMER A. The effect of a high water intake on the kidney's ability to concentrate the urine in man. J Physiol. 1957 Nov 14;139(1):42–52. doi: 10.1113/jphysiol.1957.sp005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Knapp H. R., Oelz O., Oates J. A. Stimulation of prostaglandin biosynthesis in the renal papilla by hypertonic mediums. Am J Physiol. 1978 Jan;234(1):F64–F67. doi: 10.1152/ajprenal.1978.234.1.F64. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Alterations in rat renal cortical and medullary guanosine 3'5'-monophosphate accumulation by oxygen- and calcium-dependent and -independent mechanisms: evidence for a calcium-independent action of oxygen in renal inner medulla. Metabolism. 1978 Jul;27(7):855–868. doi: 10.1016/0026-0495(78)90220-2. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Hormonal modulation of cyclic adenosine 3',5'-monophosphate-dependent protein kinase activity in rat renal cortex. Specificity of enzyme translocation. J Clin Invest. 1976 Jun;57(6):1442–1450. doi: 10.1172/JCI108414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. Reduced sensitivity of the hepatic adenylate cyclase-cyclic AMP system to glucagon during sustained hormonal stimulation. J Clin Invest. 1976 Feb;57(2):435–443. doi: 10.1172/JCI108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Craven P. A., Davis B. B. Modulation of the cyclic AMP content of rat renal inner medulla by oxygen: possible role of local prostaglandins. J Clin Invest. 1976 Dec;58(6):1370–1378. doi: 10.1172/JCI108592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Regulation of protein kinase by vasopressin in renal medulla in situ. Am J Physiol. 1977 Jan;232(1):F50–F57. doi: 10.1152/ajprenal.1977.232.1.F50. [DOI] [PubMed] [Google Scholar]
- Dousa T. P. Effect of renal medullary solutes on vasopressin-sensitive adenyl cyclase. Am J Physiol. 1972 Mar;222(3):657–662. doi: 10.1152/ajplegacy.1972.222.3.657. [DOI] [PubMed] [Google Scholar]
- EPSTEIN F. H., KLEEMAN C. R., HENDRIKX A. The influence of bodily hydration on the renal concentrating process. J Clin Invest. 1957 May;36(5):629–634. doi: 10.1172/JCI103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H. Disorders of renal concentrating ability. Yale J Biol Med. 1966 Dec;39(3):186–195. [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- George E. R., Balakir R. A., Filburn C. R., Sacktor B. Cyclic adenosine monophosphate-dependent and -independent protein kinase activity of renal brush border membranes. Arch Biochem Biophys. 1977 Apr 30;180(2):429–443. doi: 10.1016/0003-9861(77)90057-1. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Hamberg M., Samuelsson B. Inhibition of basal and hormone-stimulated adenylate cyclase in adipocyte ghosts by the prostaglandin endoperoxide prostaglandin H2. J Biol Chem. 1975 Aug 25;250(16):6460–6463. [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- KRAMER K., THURAU K., DEETJEN P. [Hemodynamics of kidney medullary substance. Part I. Capillary passage time, blood volume, circulation, tissue hematocrit and oxygen consumption of kidney medullary substance in situ]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:251–269. [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- LEVITIN H., GOODMAN A., PIGEON G., EPSTEIN F. H. Composition of the renal medulla during water diuresis. J Clin Invest. 1962 May;41:1145–1151. doi: 10.1172/JCI104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law R. O. The inulin space, solute concentrations, and weight changes in rat renal medullary slices incubated in iso-osmolal media, and their modification during anoxia and hypothermia. J Physiol. 1975 May;247(1):37–54. doi: 10.1113/jphysiol.1975.sp010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum G. M., Aisenbrey G. A., Dunn M. J., Berl T., Schrier R. W., McDonald K. M. In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin. J Clin Invest. 1977 Jan;59(1):8–13. doi: 10.1172/JCI108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. M. Purification and properties of a protein kinase from bovine corpus luteum that is stimulated by cyclic adenosine 3',5'-monophosphate and luteinizing hormone. J Biol Chem. 1973 Jan 25;248(2):494–501. [PubMed] [Google Scholar]
- RENNIE D. W., REEVES R. B., PAPPENHEIMER J. R. Oxygen pressure in urine and its relation to intrarenal blood flow. Am J Physiol. 1958 Oct;195(1):120–132. doi: 10.1152/ajplegacy.1958.195.1.120. [DOI] [PubMed] [Google Scholar]
- Rangel-Aldao R., Rosen O. M. Dissociation and reassociation of the phosphorylated and nonphosphorylated forms of adenosine 3':5' -monophosphate-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1976 Jun 10;251(11):3375–3380. [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- SAIKIA T. C. COMPOSITION OF THE RENAL CORTEX AND MEDULLA OF RATS DURING WATER DIURESIS AND ANTIDIURESIS. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:146–157. doi: 10.1113/expphysiol.1965.sp001777. [DOI] [PubMed] [Google Scholar]
- STAHL W. M. EFFECT OF MANNITOL ON THE KIDNEY: CHANGES IN INTRARENAL HEMODYNAMICS. N Engl J Med. 1965 Feb 25;272:382–386. doi: 10.1056/NEJM196502252720801. [DOI] [PubMed] [Google Scholar]
- Sakai M., Matsushita S., Nakano T., Kimura N., Araki N. Effects of parathyroid hormone in vivo on the protein kinase activity in rat kidney. Endocrinology. 1976 Jun;98(6):1443–1450. doi: 10.1210/endo-98-6-1443. [DOI] [PubMed] [Google Scholar]
- Schwartz I. L., Shlatz L. J., Kinne-Saffran E., Kinne R. Target cell polarity and membrane phosphorylation in relation to the mechanism of action of antidiuretic hormone. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2595–2599. doi: 10.1073/pnas.71.7.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THURAU K. RENAL HEMODYNAMICS. Am J Med. 1964 May;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Levitt M. J., Davis B. B. Effect of oxygen and solute on PGE and PGF production by rat kidney slices. Prostaglandins. 1977 Jan;13(1):143–151. doi: 10.1016/0090-6980(77)90051-x. [DOI] [PubMed] [Google Scholar]
- Zins G. R. Renal prostaglandins. Am J Med. 1975 Jan;58(1):14–24. doi: 10.1016/0002-9343(75)90528-8. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Clin Invest. 1977 Jul;60(1):215–223. doi: 10.1172/JCI108758. [DOI] [PMC free article] [PubMed] [Google Scholar]


