Abstract
Purpose
Oxidative stress is implicated in pancreatic β-cell dysfunction, yet clinical outcomes of antioxidant therapies on diabetes are inconclusive. Since reactive oxygen species (ROS) can function as signaling intermediates for glucose-stimulated insulin secretion (GSIS), we hypothesize that exogenously boosting cellular antioxidant capacity dampens signaling ROS and GSIS.
Methods
To test the hypothesis, we formulated a mathematical model of redox homeostatic control circuit comprising known feedback and feedforward loops and validated model predictions with plant-derived antioxidant sulforaphane (SFN).
Results
SFN acutely (30-min treatment) stimulated basal insulin secretion in INS-1(832/13) cells and cultured mouse islets, which could be attributed to SFN-elicited ROS as N-acetylcysteine or glutathione ethyl ester suppressed SFN-stimulated insulin secretion. The mathematical model predicted an adapted redox state characteristic of strong induction of endogenous antioxidants but marginally increased ROS under prolonged SFN exposure, a state that attenuates rather than facilitates glucose-stimulated ROS and GSIS. We validated the prediction by demonstrating that although 24-h treatment of INS-1(832/13) cells with low, non-cytotoxic concentrations of SFN (2-10 μM) protected the cells from cytotoxicity by oxidative insult, it markedly suppressed insulin secretion stimulated by 20 mM glucose.
Conclusions
Our study indicates that adaptive induction of endogenous antioxidants by exogenous antioxidants, albeit cytoprotective, inhibits GSIS in β-cells.
Keywords: sulforaphane, insulin secretion, ROS, Nrf2, antioxidant, computational model
Introduction
Insulin-producing pancreatic β-cells are the most abundant and important cell type in the islet of Langerhans. Autoimmune destruction of β-cells with consequent absolute insulin deficiency results in Type 1 Diabetes (T1D) (1). The development of Type 2 Diabetes (T2D) is usually associated with a combination of insulin resistance and pancreatic β-cell dysfunction involving relative insulin deficiency (2, 3). Therefore, impairment of pancreatic β-cell function is a primary contributor to the development of both types of diabetes. Although the precise pathogenic mechanisms of β-cell dysfunction are not completely understood, there is compelling evidence that oxidative stress, as a result of human exposure to environmental and dietary factors, plays a prominent role (1-5).
If unchecked, oxidative stress can considerably increase intracellular reactive oxygen species (ROS) levels, leading to oxidative damage to proteins, nucleic acids and fatty acids. Oxidative damage is believed to be a major contributing factor to the development of pancreatic β-cell dysfunction (6) and of insulin resistance in the adipose tissue, liver and skeletal muscle (7). As a result, antioxidant supplementation has been arduously advocated as a preventive and therapeutic measure to combat oxidative stress implicated in diabetes as well as a variety of other diseases. However, clinical antioxidant therapy is not as effective as expected for many disease endpoints including diabetes (8-17). While the observed ineffectiveness of antioxidant supplements may be attributed to many factors, including trial design, dose, frequency and bioavailability, these clinical data cast serious doubt on their usefulness.
Although cytotoxic at higher levels, ROS also function as intracellular second messengers. For instance, ROS derived from glucose metabolism act as a necessary intermediate signal to mediate glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells and glucose sensing in hypothalamic neurons (18-23). The involvement of ROS as a signaling intermediate suggests that its magnitude would be inversely correlated with the ROS-scavenging capacity in the cell. This notion conceivably creates a conundrum for the effect of exogenous antioxidants on β-cell function. On the one hand, exogenous antioxidants can be cytoprotective against oxidative damage. On the other hand, they may indiscriminately attenuate glucose-stimulated ROS accumulation and thus GSIS. The latter may well explain why antioxidant supplementation has largely failed in preventing and treating diabetes, especially given that antioxidant supplements are often taken at doses several times higher than recommended (24).
Sulforaphane (SFN) is a dietary isothiocyanate from cruciferous vegetables, with an especially high content in broccoli (25). SFN has been proven as an effective chemopreventive agent (26, 27). In β-cells, SFN can protect against cytokine- and streptozotocin-induced cell damage (28). Chemically as weak pro-oxidant, SFN has been demonstrated to promote ROS generation in several cell types (29-31) and induce expression of multiple antioxidant and phase II enzymes through activating the canonical nuclear factor E2-related factor 2 (Nrf2) pathway (25, 32). Because SFN and other similar phytochemical antioxidants can increase both intracellular ROS and antioxidant capacity (33, 34), their effects on physiological second messenger ROS and insulin secretion are not straightforward, which may depend on the dynamical response of the cellular redox homeostatic system.
The redox homeostatic circuit is generally structured as a negative feedback loop comprising ROS, sensor protein Keap1, transcription factor Nrf2 and antioxidant enzymes (35). Indirect antioxidant phytochemicals such as SFN are able to directly modify cysteine residues of the sensor protein Keap1 to activate Nrf2 (36-38), thus forming an important feedforward loop that induces endogenous antioxidants (Fig. 1). Under chronic exposure to exogenous Nrf2-activating phytochemicals such as SFN, the redox system will adapt and settle to a steady state. We propose that due to the nonlinearity and signal amplification associated with the feedforward and feedback regulation, the steady-state antioxidant capacity would generally rise to a great extent, and as a result, the increase in basal ROS is very limited (39). This adapted redox state is likely to favor attenuation rather than facilitation of glucose-stimulated ROS signals and thus GSIS (35). In the present study, we formulated a mathematical model of a simplified redox homeostatic circuit to formally test the hypothesis. We verified the model predictions with SFN as a prototype antioxidant in INS-1(832/13) cells and isolated mouse islets. We found, as the model predicts, that SFN acutely stimulates basal insulin secretion in pancreatic β-cells, which can be attributed to initially uncompensated ROS production. In contrast, prolonged SFN treatment only has a marginal stimulatory effect on basal insulin secretion. More importantly, prolonged SFN treatment activates Nrf2 and the adaptive antioxidant response, which despite protecting the cells from oxidative insult, suppresses GSIS.
Figure 1. A general molecular circuit controlling cellular redox homeostasis in response to perturbation by SFN.
The ROS level is regulated through both negative feedback (lower) and incoherent feedforward (upper) loops. The ubiquitination activity of Keap1 is inhibited by ROS and SFN, allowing stabilization of Nrf2. Nrf2 then induces antioxidant genes, which collectively function to eliminate ROS.
Materials and Methods
Cell culture and reagents
INS-1(832/13) cells were kindly provided by Dr. Christopher Newgard (Duke University) and were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS), 10 mM glucose, 25 mM HEPES, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. Passages 55-59 at 75-90% confluence were used for the current study. There was no difference in the glucose responsiveness of the cells among different passages. Culture media, fetal bovine serum (FBS), supplements and TRIzol were purchased from Invitrogen (Carlsbad, CA). SFN, fatty acid-free bovine serum albumin (BSA), β-mercaptoethanol, glucose oxidase (GO), diethylmaleate (DEM), N-acetylcysteine (NAC), glutathione ethyl ester (GSH-EE), tert-Butylhydroquinone (tBHQ), potassium chloride (KCl) and glucose solution (45%) were obtained from Sigma (St. Louis, MO).
Islets isolation and primary culture
Pancreatic islets were isolated from 9 to 12-week-old C57BL/6J mice (The Jackson Laboratories, Bar Harbor, ME) by collagenase P (Roche, Switzerland) digestion, as previously described (21). Islets were picked by hand four times in succession under a dissecting microscope and cultured 48 hr in RPMI 1640 supplemented with 10 mM glucose, 10% FBS, 25 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. All procedures were performed in accordance with the Institutional Guidelines for Animal Care at The Hamner Institutes for Health Sciences.
Measurement of insulin secretion
Insulin secretion in INS-1(832/13) cells and isolated islets were performed in static incubation as described previously (40). Insulin contents in the media were determined using an RIA kit (Linco Research, St. Charles, MO) with rat insulin as the standard. Levels of secreted insulin by INS-1(832/13) cells were normalized to DNA content, which was determined by an overnight incubation at 42 °C with a lysis buffer containing 30 mM Tris-HCl, 10 mM EDTA, 1% SDS and 50 μg/ml proteinase K (Qiagen, Valencia, CA), followed by a measurement of absorbance at 260 nm using a Nanodrop spectrophotometer (Thermo Scientific, Inc).
Intracellular peroxide determination
Intracellular peroxide levels in INS-1(832/13) cells were measured by flow cytometry (Becton Dickinson FACSCanto II, Becton Dickinson, San Jose, CA) using the fluorescent probe 5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Invitrogen) as described previously (21). The final concentration of CM-H2DCFDA used was 2 μM and loading time was 30 min.
Antioxidant response element reporter assay
Cignal Lenti antioxidant response element (ARE) reporter lentiviral particles were obtained from SABiosciences (Frederick, MD). Lentiviral transduction of INS-1(832/13) cells was performed as described previously (40, 41). Cells were grown to ∼90% confluency and sub-cultured in medium containing 0.35 μg/ml of puromycin. The luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's protocol. The luciferase activity was normalized to cell viability which was determined using a Non-Radioactive Cell-Proliferation Assay Kit (Promega).
Quantitative real-time RT–PCR analysis
Total RNA was isolated with TRIzol (Invitrogen) and subsequently subjected to cleanup using an RNase-Free DNase Set and RNeasy Mini kit (Qiagen, Valencia, CA). Quantitative real-time RT-PCR was performed as described previously (42). The primers (sequences are shown in Table S1, Supplementary Materials) were designed using Primer Express 4 (Applied Biosystems, Foster City, CA) and synthesized by MWG-BIOTECH Inc. (High Point, NC). Real-time fluorescence detection was performed using an ABI PRISM 7900 HT Fast Real-time PCR System (Applied Biosystems).
Western blot analysis
Isolation of cell fractions and western blotting were performed as detailed previously (41, 43). The antibody for Nrf2 (sc-13032; 1:500) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibodies for β-ACTIN (A1978; 1:2000), LAMIN A (L1293; 1:2,500) and α-TUBULIN (T5168; 1:2,000) were purchased from Sigma.
Cell viability
A minimum of five replicates of 10,000 cells/well were plated in 96-well plates and allowed to adhere to the plate for 24 h, after which the medium was removed and replaced with fresh medium containing vehicle or SFN. Cells viability was determined using the CellTiter Non-Radioactive Cell-Proliferation Assay Kit with MTS [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Promega, Madison, WI) as described previously (44). Measurements are expressed as a percentage of untreated control cells.
Statistical analyses
All statistical analyses were performed using Graphpad Prism 4 (GraphPad Software, San Diego, CA) with p < 0.05 taken as significant. More specific indices of statistical significance are indicated in individual figure legends. The data are expressed as mean ± SD. For comparisons among groups, a one-way or two-way ANOVA with Bonferroni post hoc testing was performed.
Model formulation
The mathematical model is primarily based on the molecular circuit schematically illustrated in Fig. 1. In the model, ROS are treated collectively as a single state variable, and antioxidant genes/enzymes also are treated as a single state variable named as AC, which represents the overall cellular Antioxidant Capacity. Because the role of sensor molecule Keap1 is to promote Nrf2 degradation, for simplicity, it is reasonable to omit Keap1 and assume that ROS and SFN directly inhibit the degradation process of Nrf2. Nrf2 then transcriptionally upregulates AC and AC is responsible for increasing the clearance of ROS. The following four ordinary differential equations describe the redox control circuit, its perturbation by SFN, as well as ROS- and glucose-stimulated insulin secretion. The state variables ROS, Nrf2, AC and insulin have arbitrary unit. The model was parameterized such that the steady-state levels of ROS, Nrf2, and AC are unity at basal conditions where SFN=0 and glucose=3 mM. The amount of insulin secreted in 30 min at the above condition was also parameterized to unity. All parameter values are listed in Table S2 (Supplementary Materials). The model was constructed in Berkeley Madonna (University of California, Berleley, CA) and solved with the Rosenbrock stiff solver.
| (1) |
| (2) |
| (3) |
| (4) |
Results
Cellular redox homeostatic control is a dynamical process involving multiple transcriptional controls and antioxidant genes. Despite the complexity, its qualitative behavior as a dynamical control system can be rationalized by a general circuit structure involving both feedback and feedforward regulations (35). By formulating a simple mathematical model for this circuit, as illustrated in Fig. 1, we analyzed the dynamical and steady-state changes in ROS and antioxidant levels induced by Nrf2-activating antioxidant SFN. Qualitative predictions made with the model on basal and glucose-stimulated insulin secretion were then validated in cultured β-cells and/or mouse islets.
Adaptive response of simulated redox homeostatic control circuit
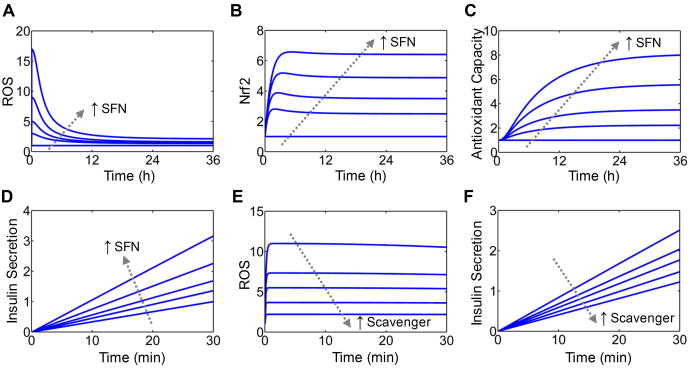
The simulated dynamical responses of ROS, Nrf2 and AC to perturbations by various concentrations of SFN follow a typical adaptive response pattern (Fig. 2A-2C). Upon SFN challenge, the ROS level spikes immediately and gradually decreases over time till settling to a new steady state slightly above the baseline (Fig. 2A). This adaptive change in ROS occurs because Nrf2 is first activated by ROS through the feedback loop and by SFN through the feedforward loop (Fig. 2B), then it upregulates AC (Fig. 2C) to remove ROS until reaching a lower steady state.
Figure 2. Simulated dynamical responses to SFN.
(A-D) Simulated time courses of ROS, Nrf2, AC and basal insulin secretion in response to continuous exposure to SFN at various concentrations (0, 4, 8, 16 and 32 μM). Except for insulin secretion, shown are fold increases over baseline levels. For insulin secretion, shown are the amount accumulated in the first 30 min of SFN treatment normalized to the accumulated amount for SFN=0. (E and F) Simulated effects of increasing ROS-scavenging activity on transient ROS and basal insulin secretion stimulated by SFN. Shown are simulations with scavenger at level 0, 0.5, 1, 2, 4 in response to 20 μM SFN.
Effect of acute SFN treatment on basal insulin secretion
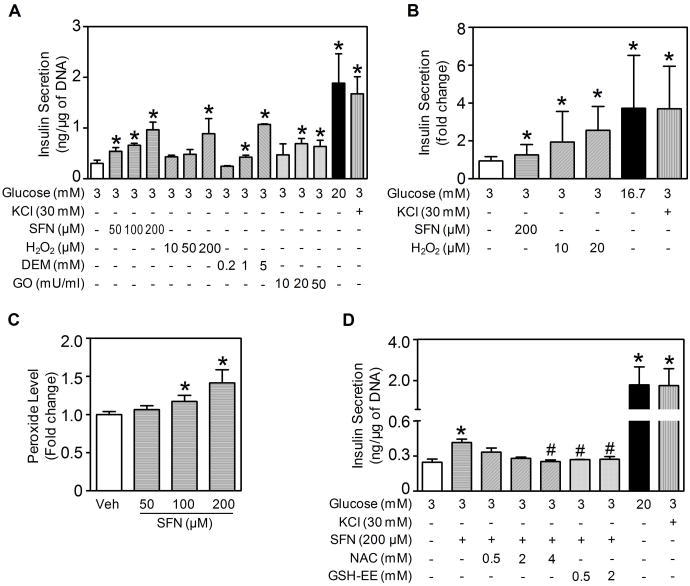
Because ROS can stimulate insulin secretion (18-22), the transient increase in ROS immediately following the onset of SFN treatment (Fig. 2A) is expected to acutely boost basal insulin secretion. The model showed that in the presence of low glucose (3 mM), secreted insulin accumulates to higher levels following SFN stimulation in a concentration-dependent manner (Fig. 2D). This acute, stimulatory effect of SFN was first validated in INS-1(832/13) cells, a cell line widely used to evaluate β-cell function in vitro(45). As shown in Fig. 3A, exposing the cells to SFN for 30 min concentration-dependently stimulated insulin secretion while glucose was kept at the basal level (3 mM). Other oxidative secretagogues, including hydrogen peroxide (H2O2), DEM and GO, produced similar stimulatory effects. DEM stimulates insulin secretion by depleting sulfhydryl to enhance ROS accumulation (21), whereas GO oxidizes glucose to generate H2O2. The stimulatory effect of acute SFN treatment also occurred with isolated mouse islets, where a modest but statistically significant increase in basal insulin secretion was observed (Fig. 3B). As expected, H2O2 also potently stimulated insulin release from the islets. As positive control, high-level glucose and KCl both readily stimulated insulin secretion in INS-1(832/13) cells and isolated mouse islets (Fig. 3A and 3B).
Figure 3. Acute effect of SFN on basal insulin secretion and involvement of ROS.
(A) Insulin secretion by INS-1(832/13) cells treated with SFN or other oxidative agents (H2O2, DEM and GO) at various indicated concentrations for 30 min under 3 mM glucose condition. High glucose (20 mM) and KCl were used as positive controls. Secreted insulin was normalized by DNA content. (B) Insulin secretion by isolated mouse islets treated with SFN or H2O2 at indicated concentrations for 30 min under 3 mM glucose condition. High glucose (16.7 mM) and KCl were used as positive controls. Secreted insulin was normalized by islet number. For panels (A) and (B), n = 3 - 6 independent experiments. *, p < 0.05 vs. 3 mM glucose alone. (C) SFN-stimulated intracellular peroxide production. INS-1(832/13) cells were challenged with SFN at various indicated concentrations for 30 min under 3 mM glucose condition. Veh, Vehicle (Kreb's buffer with 3 mM glucose). n = 3; *, p < 0.05 vs. Veh. (D) Pretreatment of INS-1(832/13) cells with ROS-scavenging antioxidants NAC or GSH-EE suppressed SFN-stimulated insulin secretion. Cells were pretreated with NAC or GSH-EE at indicated concentrations for 30 min followed by SFN stimulation for additional 30 min under 3 mM glucose condition. *, p < 0.05 vs. 3 mM glucose alone; #, p < 0.05 vs. 200 μM SFN at 3 mM glucose.
To determine whether the stimulation of insulin secretion by SFN involves ROS as a signaling intermediate, intracellular ROS levels in INS-1(832/13) cells were determined. As shown in Fig. 3C, 30-min SFN exposure concentration-dependently increased intracellular peroxide levels as measured by using CM-H2DCFDA. To determine whether the observed increase in ROS by SFN was indeed involved in stimulating insulin secretion, we examined the effects of SFN in the presence of ROS-scavenging agents. Both NAC and GSH-EE blocked SFN-stimulated insulin secretion from INS-1(832/13) cells (Fig. 3D). The mathematical model was also able to recapitulate this phenomenon, showing that increasing the rate of ROS scavenging dampened the transient increase in ROS stimulated by SFN (Fig. 2E) and consequently insulin secretion (Fig. 2F). Taken together, these results demonstrated that acute SFN treatment stimulates basal insulin secretion in β-cells, which is mediated, at least in part, by SFN-generated ROS.
Effects of chronic SFN treatment on insulin secretion
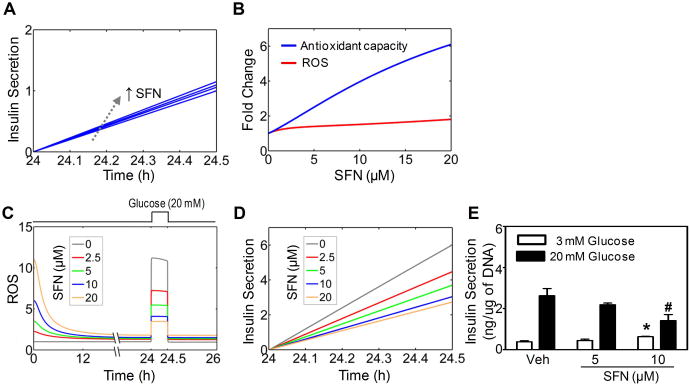
While the acute, stimulatory effect of SFN on basal insulin secretion can be readily explained by the initial transient ROS accumulation, the consequence of prolonged exposure to SFN is not as straightforward. As shown in Fig. 2A-2C, cells persistently exposed to SFN are expected to have adapted and reached a new steady state, where Nrf2 and AC are markedly elevated but with only slightly increased ROS levels. This adapted state is likely to exert differential effects on basal insulin secretion vs. GSIS. The slightly increased ROS at the adapted state would only marginally boost basal insulin secretion, according to model simulations (Fig. 4A). This prediction was confirmed with INS-1(832/13) cells, which showed that continuous treatment with SFN at non-cytotoxic concentrations for 24 h caused only a fractional increase in basal insulin secretion under 3 mM glucose (Fig. 4E).
Figure 4. Chronic effects of SFN on basal insulin secretion and GSIS.
(A) Simulated basal insulin secretion in the last 30 min of continuous exposure to SFN at various concentrations (0, 2, 5, 10, and 20 μM) for 24.5 h (B) Simulated dose responses of antioxidant capacity and ROS following continuous exposure to SFN for 24 h. (C) Simulated attenuation of glucose-stimulated ROS signal by SFN. SFN is present continuously for 26 h and glucose is switched from 3 to 20 mM at 24 h and back to 3 mM at 24.5 h as indicated by the square wave on top. (D) Simulated GSIS during the 30 min (24 – 24.5 h) when glucose is at 20 mM as in (C). (E) Experimentally measured basal insulin secretion (white bar) or GSIS (black bar) in INS-1(832/13) cells following 24-h SFN exposure at concentrations indicated. Secreted insulin was normalized by DNA content. n = 3 - 6 independent experiments; *, p < 0.05 vs. Veh with 3 mM glucose; #, p < 0.05 vs. Veh with 20 mM glucose.
In the context of ROS functioning as signaling molecules, the effect of prolonged SFN exposure on GSIS depends on the direction in which glucose-stimulated ROS are modulated at the adapted redox state. For the circuit in Fig. 1, the incoherent feedforward loop and potential ultrasensitive signaling motifs embedded in the feedback loop can amplify the induction of antioxidant enzymes by SFN, keeping the ROS level in check (35, 39). The mathematical model recapitulated the final adapted state, which is characterized by a marginal increase in ROS but a higher fold increase in AC for a range of SFN concentrations (Fig. 4B), a state consistent with the antioxidant role of SFN. The small increase in basal ROS is expected to have little impact on glucose-stimulated ROS signal. In contrast, the large fold induction of AC is likely to markedly attenuate glucose-stimulated ROS signal. The model presaged that with prolonged exposure to SFN, the transient ROS signal stimulated by 20 mM glucose can be significantly attenuated, and the higher the SFN concentration, the smaller the glucose-stimulated ROS signal (Fig 4C). Correspondingly, GSIS was predicted to be suppressed by SFN in a concentration-dependent manner (Fig. 4D).
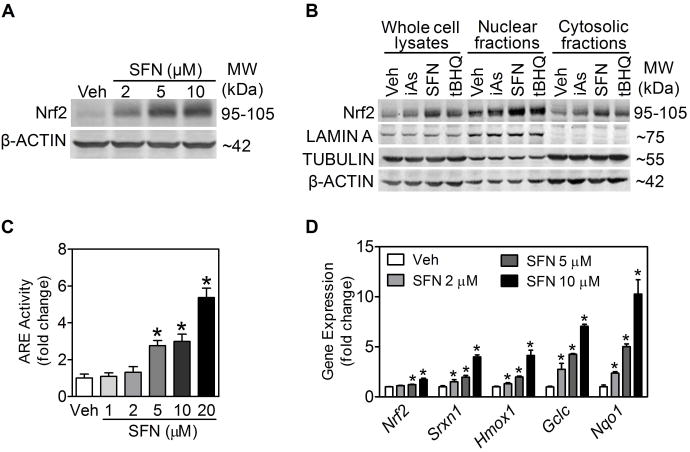
We sought to verify the model predictions with INS-1(832/13) cells. Although activation of Nrf2-mediated antioxidant response by SFN has been reported in many cell types, including pancreatic β-cells (28), it is unknown whether the response indeed occurs in INS-1(832/13) cells. As shown in Fig. 5A, 6-h SFN treatment markedly increased cellular Nrf2 protein levels in a concentration-dependent fashion. As with other well-known Nrf2 activators, such as arsenite and tert-butylhydroquinone (tBHQ), SFN resulted in a substantial nuclear accumulation of Nrf2 protein (Fig. 5B). In addition, INS-1(832/13) cells stably transfected with an ARE-luciferase reporter showed concentration-dependent induction of luciferase activity following SFN treatment (Fig. 5C). Lastly, expression of antioxidant genes, such as sulfiredoxin 1 (Srxn1), heme oxygenase 1 (Hmox1), γ-glutamate cysteine ligase catalytic subunit (Gclc) and NAD(P)H: quinone oxidoreductase 1 (Nqo1) were all significantly induced by SFN (Fig. 5D).
Figure 5. Activation of Nrf2 and antioxidant response by SFN in INS-1(832/13) cells.
(A) SFN concentration-dependently increased Nrf2 protein levels in INS-1(832/13) cells. Cells were treated with SFN for 6 h, and whole-cell lysates were used for immunoblotting. (B) SFN increased Nrf2 protein accumulation in nuclear fractions. Whole cell lysates, nuclear fractions and cytosolic fractions were collected after treatment with Veh (medium) and the indicated agents for 6 h. iAs, arsenite (5 μM); SFN (10 μM); tBHQ, tert-butylhydroquinone (50 μM). LAMIN A, TUBULIN and β-ACTIN were used as loading controls for nuclear fractions, cytosolic fractions and whole cell lysates, respectively. (C) SFN concentration-dependently induced ARE-luciferase activity. Cells were treated with SFN for 9 h. n = 4; *, p < 0.05 vs. Veh (medium). (D) Transcripts of Nrf2 and ARE-dependent genes measured by real-time RT-PCR. Cells were treated with SFN for 6 h. n = 2 - 5 independent experiments; *, p < 0.05 vs. Veh.
With the knowledge that SFN indeed activates Nrf2 and the adaptive antioxidant response in INS-1(832/13) cells, we then sought to test the model's prediction on the effect of chronic SFN treatment on GSIS. For INS-1(832/13) cells that were continuously incubated with SFN at low, non-cytotoxic concentrations for 24 h, insulin secretion stimulated by 20 mM glucose was significantly attenuated in comparison to control cells, and a higher SFN concentration resulted in a more significant suppression of GSIS (Fig. 4E). This result is highly consistent with the model prediction shown in Fig. 4D. The attenuation of GSIS is not likely to result from cytotoxic damage to the insulin secretory apparatus by SFN because the SFN concentrations used were non-cytotoxic (Fig. 6A), and more over, basal insulin secretion was not compromised, but rather slightly increased at the same concentrations (Fig. 4E). To further prove that SFN at low concentrations is indeed antioxidant and cytoprotective, we pretreated INS-1(832/13) cells with 2 μM SFN for 24 h and subsequently exposed them to various concentrations of H2O2 for an additional 6 h. Pretreatment with SFN led to a significantly increased resistance to cellular toxicity by H2O2, as evidenced by the marked right-ward shift in the cell viability curve (Fig. 6B). In accordance with the indirect antioxidant role of SFN, the cytoprotective effects likely resulted from the induction of endogenous antioxidants at low concentrations of SFN (Fig. 5D).
Figure 6. Low, non-cytotoxic concentrations of SFN protected cells from H2O2-induced cytotoxicity.
(A) Cytotoxicity of SFN in INS-1(832/13) cells. Cell viability was measured with the MTT assay following SFN treatment for 24 h. (B) INS-1(832/13) cells were pretreated with 2 μM SFN or Vehicle (medium) for 24 h, followed by H2O2 exposure for 6 h and measurement of cell viability. n = 4; *, p < 0.05 vs. Vehicle with the same-concentration H2O2 treatment.
Discussion
Oxidative damage of pancreatic β-cells by excessive ROS has been regarded as a key pathogenic factor for β-cell failure (6). Recent advances in β-cell study have revealed a physiological role for ROS, in particular H2O2, in mediating GSIS (18-22). This paradox makes evaluating the effects of redox-altering compounds on insulin secretion a challenging task. Further complicating the matter is the fact that many redox-altering compounds can be either pro- or anti-oxidants, depending on the concentrations and duration applied, as the cellular redox balance responds dynamically to the chemical perturbations. In the present study, we dissected the effects of SFN, a typical plant-derived Nrf2-activating antioxidant, on both basal and glucose-stimulated insulin secretion. We utilized a mathematical model to capture the dynamical changes of ROS and antioxidant capacity in the process of adaptive antioxidant induction and redox homeostatic control. The integrated study reveals that an acute challenge of INS-1(832/13) cells or cultured mouse islets with SFN triggers insulin secretion, which is mediated by SFN-stimulated transient ROS accumulation. Conversely, due to activation of Nrf2 and induction of downstream antioxidant enzymes, prolonged SFN exposure attenuates glucose-stimulated ROS and GSIS, albeit protecting the cells from future oxidative insults. The findings suggest that as an Nrf2-activator, SFN may unnecessarily stimulate basal insulin release during fasting when insulin is not needed, but inhibit postprandial GSIS when insulin is needed for maintaining blood glucose levels.
Growing evidence indicates that ROS can function as second messengers that serve an intracellular signaling role in a variety of organs or tissues involved in glucose metabolism (46, 47). In addition to pancreatic β-cells (21, 22), H2O2 as a second messenger is involved in insulin signaling in the liver, adipose tissue and skeletal muscle where glucose uptake takes place, and in glucose sensing in the hypothalamus where feeding behavior is regulated (23, 48, 49). Given the physiological role of ROS in these multiple processes regulating glucose catabolism, it is reasonable to argue that unduly boosting intracellular antioxidant levels may have pathogenic consequences with the development of diabetes. In keeping with this notion, it was demonstrated that mice overexpressing H2O2-metabolizing enzyme glutathione peroxidase or catalase exhibit decreased insulin sensitivity, impaired β-cell function, and accelerated development of obesity and diabetes (50, 51).
The conventional view of ROS as a pathogenic factor has led to the advocacy of antioxidant supplementation to promote general health, including preventing and treating diabetes. Although certain clinical studies with antioxidant supplements showed health benefits in a limited number of settings such as cancer prevention (52), substantial evidence indicates antioxidant therapy is not as effective as expected for many disease endpoints including diabetes (8-17). While the ineffectiveness may be attributed to many clinical factors, by cancelling out the benefit of cytoprotection provided by exogenous antioxidants, the inhibitory action of antioxidants on GSIS, as observed here in vitro with SFN, as well as the potential inhibition by antioxidants of insulin action in the adipose tissue and liver, and of glucose-sensing in the hypothalamus, could all contribute to the observed ineffectiveness,
While the inhibitory effect of SFN on GSIS observed here in vitro is attributed to enhanced antioxidant capacity, SFN may also perturb other signaling pathways that are involved in GSIS. One candidate pathway is NF-κB, whose transcriptional activity appears to be necessary for GSIS, as attenuation of NF-kB activation disrupted the expression of genes involved in glucose uptake, oxidative metabolism, and insulin exocytosis (53-55). A number of studies have demonstrated that SFN and other isothiocyanate derivatives can inhibit the transcriptional activation of NF-κB (56, 57). Therefore it is likely that inhibition of GSIS by SFN may also be mediated in part by suppression of NF-κB. In addition, the NF-κB-mediated transcriptional response plays a crucial role in regulating β-cell proliferation and apoptosis in response to cytokines involved in inflammation, with the outcome dependent on the magnitude and duration of NF-κB signaling (58-61). The anti-inflammatory function of SFN through suppressing NF-κB suggests that SFN may have an effect on β-cell fate in islets in vivo, by which insulin secretion can be altered.
GSIS is regulated by the rate of glucose metabolism within β-cells. Increased glucose leads to an increase in the glycolytic flux and an acceleration of mitochondrial NADH production. Oxidation of NADH increases ATP synthesis, decreases ADP concentration and thus increases ATP/ADP ratio. These processes are critically important for insulin secretion. Our previous studies demonstrated that ROS alone cannot increase insulin secretion without ATP (21), which suggests that the effects of SFN on insulin secretion may eventually rely on changes in the ATP/ADP ratio. In addition, shuttling of glycolysis-derived NADH into mitochondria has been shown to play important role in mediating GSIS (62). It appears that SFN may increase the NAD(P)H/NAD(P) ratios, as shown in other cell types (63, 64). However, it remains to be seen whether the effects of SFN, acutely or chronically, on insulin secretion can be mediated in part through perturbing the ratios of the redox pairs in β-cells.
Given the common antioxidant mechanism for many plant-derived Nrf2-activating compounds, such as curcumin, catechin, resveratrol, and oleanolic acid, it is tempting to surmise that these compounds may dysregulate insulin secretion in a similar manner as SFN, especially if their primary action is on the cellular redox system. Numerous studies using cultured β-cells or isolated animal islets seem to support that these compounds are largely stimulatory to basal insulin secretion (65-69). Yet their effects on GSIS are much less consistent; both augmentation and suppression of GSIS by these compounds have been observed in vitro(65, 67, 69-73). While time and compound concentration used in these studies are variables that may explain some of these discrepancies, another important possibility is that many of these compounds can regulate processes other than Nrf2 activation. For instance, the inhibition of GSIS by resveratrol has been attributed to multiple processes that resveratrol may affect, including blockade of voltage-gated Ca2+ channels and shifting of glucose metabolism from mitochondrial oxidation to anaerobic catabolism (71, 74). Therefore the final outcome of Nrf2-activating compounds on GSIS is likely the result of combined effects of multi-pathway modulation by these compounds. While much of information is available for the effects of Nrf2 activators on insulin secretion, the consequence of Nrf2 inhibition has not been explored. Recently it was discovered that brusatol, extracted from Brucea javanica (L) Merr., can inhibit Nrf2 by enhancing its ubiquitination and proteasomal degradation (75). By increasing the degradation rate constants of Nrf2 to mimic the effect of brusatol, our model simulation showed that basal Nrf2 level and antioxidant capacity are lowered and basal ROS level is increased. As a result, the model predicted that in the presence of brusatol, basal insulin secretion is elevated, but contrary to the effect of SFN, GSIS is also augmented (simulation results not shown). Such predictions remain to be validated by further experimental studies.
As demonstrated in the present study, SFN can protect β-cells from the cytotoxicity induced by exogenous oxidative stressors such as H2O2. However, the surviving β-cells are not necessarily functioning equally well as non-stressed cells. The induction of endogenous antioxidants in those stressed but surviving β-cells can blunt GSIS. Although effective as chemoprevention agents, the long-term health consequence of Nrf2-activating compounds in non-cancer endpoints is still unclear. The finding that prolonged SFN treatment results in attenuated GSIS in β-cells raised a reasonable concern over the application of SFN and other Nrf2-activating antioxidants in chemoprevention since insulin deficiency may become a tangible side effect. Altering the cellular redox state through antioxidant supplementation may do both good – and harm, through potentially inhibiting physiological H2O2 – thus producing no overall health benefit. It is likely difficult to strike the right balance by optimizing the dose to achieve overall beneficial results. The present study suggests that the timing of antioxidant supplementation might be a factor to be considered in order to minimize the potential side effect on insulin secretion. For instance, by taking Nrf2-activating antioxidants at meal time, it may (a) help stimulate insulin secretion given their initial ROS-generating effect and (b) minimize inhibition of GSIS because it takes several hours for endogenous antioxidants to be induced in β-cells, by which time, blood glucose would have receded and insulin is no longer needed. Nevertheless, this postulated effect of timing clearly needs further experimental investigations in whole animals.
Although SFN is generally regarded as an antioxidant because it induces endogenous antioxidant enzymes, it is quite intriguing to note that arsenite, an environmental oxidative chemical, exhibits similar divergent effects on insulin secretion. We have demonstrated previously with INS-1(832/13) cells and isolated mouse islets that arsenite acutely stimulated insulin secretion under basal glucose conditions, and prolonged exposure of these cells to non-cytotoxic arsenite markedly blunted GSIS (21, 40). The inhibitory effect on insulin secretion is due to the induction of endogenous antioxidant enzymes by arsenite, which blocks glucose-stimulated signaling ROS. We have thus proposed that adaptive induction of endogenous antioxidant enzymes, rather than oxidative damage, is the primary pathogenic mechanism by which low-level environmental oxidative stressors impair β-cell function. In light of this premise, combating environmental oxidative stress with antioxidant supplements such as SFN, which also induces endogenous antioxidant genes, can be futile if not worsening the GSIS response. Nevertheless, the cytoprotection provided by SFN and other Nrf2-activating compounds – achieved by the induction of phase II enzymes and antioxidants to promote detoxification of oxidative chemicals and their reactive metabolites – cannot be dismissed. Antioxidant compounds that can directly scavenge oxidative chemicals in extracellular compartments without interfering with intracellular signaling ROS are likely to be more effective therapeutically with minimal side effect and therefore should be developed.
Interpreting and predicting experimental results in biological systems have increasingly relied on simple but adequate mathematical models of the underlying molecular circuits. The antioxidant response model formulated here followed the principle of parsimony by excluding details of regulatory interactions and metabolic reactions catalyzed by a variety of antioxidant enzymes. Yet the nonlinearity and dynamics captured by the simplified model, which are key to our general understanding of the system, are unlikely to be qualitatively different from more complicated redox control circuits that share the same overall feedback and feedforward structure (35). Importantly, predictions made with this simple model were corroborated by experimental results.
In conclusion, this integrated study demonstrates that SFN has a paradoxical role in pancreatic β-cell function. First, SFN may stimulate basal insulin secretion through transient ROS production; Second, SFN activates Nrf2-mediated antioxidant response and protects β-cells from oxidative and electrophilic stress-induced cell damage. Third, prolonged SFN exposure may blunt ROS signaling and attenuate GSIS in pancreatic β-cells. To develop therapeutic approaches using SFN and other potent Nrf2 activators, further investigations are needed.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health Grants DK76788 (to J.P.) and ES016005 (to J.P.) and the DOW Chemical Company (to M.E.A.). The content is solely the responsibility of the authors. All authors have agreed to its content and there are no financial or other conflicts of interest. J.F., B.Y., Q.Z., C.G.W, K.Y., M.E.A. and J.P. are/were employees of The Hamner Institutes for Health Sciences. The Hamner is a 501(c)3 not-for-profit organization that has a diverse research portfolio that includes funding from the American Chemistry Council, a trade association that represents chemical manufacturers.
Abbreviations
- AC
antioxidant capacity
- ARE
antioxidant response element
- BSA
bovine serum albumin
- CM-H2DCFDA
5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate, acetyl ester
- DEM
diethylmaleate
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- GCLC
γ-glutamate cysteine ligase catalytic subunit
- GSH-EE
glutathione ethyl ester
- GSIS
glucose-stimulated insulin secretion
- HMOX-1
heme oxygenase 1
- H2O2
hydrogen peroxide
- NAC
N-acetylcysteine
- NQO1, NAD(P)H
quinone oxidoreductase 1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- ROS
reactive oxygen species
- SFN
sulforaphane
- SRXN1
sulfiredoxin 1
- tBHQ
tert-butylhydroquinone
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
References
- 1.Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, Pietropaolo M, Arvan PR, Von Herrath M, Markel DS, Rhodes CJ. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–1379. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Fridlyandand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes. 2004;53:1942–1948. doi: 10.2337/diabetes.53.8.1942. [DOI] [PubMed] [Google Scholar]
- 4.Robertsonand RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Kajimotoand Y, Kaneto H. Role of oxidative stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–176. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- 6.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(1):S119–124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 7.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 8.Ezzedine K, Latreille J, Kesse-Guyot E, Galan P, Hercberg S, Guinot C, Malvy D. Incidence of skin cancers during 5-year follow-up after stopping antioxidant vitamins and mineral supplementation. Eur J Cancer. 2010;46:3316–3322. doi: 10.1016/j.ejca.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 11.Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2003;29:579–585. doi: 10.1016/s1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- 12.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsuand CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 17.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 18.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 19.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7:38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150:2197–2201. doi: 10.1210/en.2008-1149. [DOI] [PubMed] [Google Scholar]
- 21.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 22.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Periquet A, Fernandez Y, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- 24.Chun OK, Floegel A, Chung SJ, Chung CE, Song WO, Koo SI. Estimation of antioxidant intakes from diet and supplements in U.S. adults. J Nutr. 2010;140:317–324. doi: 10.3945/jn.109.114413. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 27.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:647–652. [PubMed] [Google Scholar]
- 28.Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol. 2009;235:57–67. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 30.Moon DO, Kim MO, Kang SH, Choi YH, Kim GY. Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009;274:132–142. doi: 10.1016/j.canlet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Moon DO, Kang SH, Kim KC, Kim MO, Choi YH, Kim GY. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010;295:260–266. doi: 10.1016/j.canlet.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohleand C, Bock KW. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem Pharmacol. 2006;72:795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu C, Eggler AL, Mesecar AD, van Breemen RB. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol. 2011;24:515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom. 2007;18:2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhangand Q, Andersen ME. Dose response relationship in anti-stress gene regulatory networks. PLoS Comput Biol. 2007;3:e24. doi: 10.1371/journal.pcbi.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118:864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods CG, Fu J, Xue P, Hou Y, Pluta LJ, Yang L, Zhang Q, Thomas RS, Andersen ME, Pi J. Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol Appl Pharmacol. 2009;238:27–36. doi: 10.1016/j.taap.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang B, Fu J, Zheng H, Xue P, Yarborough K, Woods CG, Hou Y, Zhang Q, Andersen ME, Pi J. Deficiency in the nuclear factor E2-related factor 2 renders pancreatic beta-cells vulnerable to arsenic-induced cell damage. Toxicol Appl Pharmacol. 2012;264:315–323. doi: 10.1016/j.taap.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhao R, Hou Y, Xue P, Woods CG, Fu J, Feng B, Guan D, Sun G, Chan JY, Waalkes MP, Andersen ME, Pi J. Long isoforms of NRF1 contribute to arsenic-induced antioxidant response in human keratinocytes. Environ Health Perspect. 2011;119:56–62. doi: 10.1289/ehp.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newgard CB, Lu D, Jensen MV, Schissler J, Boucher A, Burgess S, Sherry AD. Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes. 2002;51(3):S389–393. doi: 10.2337/diabetes.51.2007.s389. [DOI] [PubMed] [Google Scholar]
- 46.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 47.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2011;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: reactive oxygen species may have a protective role in pancreatic beta-cells. Diabetes. 2006;55:1592–1604. doi: 10.2337/db05-1357. [DOI] [PubMed] [Google Scholar]
- 52.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 53.Norlin S, Ahlgren U, Edlund H. Nuclear factor-{kappa}B activity in {beta}-cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 54.Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller DG, Halban PA. Activation of NF-kappaB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J Biol Chem. 2005;280:30630–30637. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- 55.Bernal-Mizrachi E, Wen W, Shornick M, Permutt MA. Activation of nuclear factor-kappaB by depolarization and Ca(2+) influx in MIN6 insulinoma cells. Diabetes. 2002;51(3):S484–488. doi: 10.2337/diabetes.51.2007.s484. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 57.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 58.Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS. NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci U S A. 2007;104:1913–1918. doi: 10.1073/pnas.0610690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang I, Kim S, Kim JY, Cho N, Kim YH, Kim HS, Lee MK, Kim KW, Lee MS. Nuclear factor kappaB protects pancreatic beta-cells from tumor necrosis factor-alpha-mediated apoptosis. Diabetes. 2003;52:1169–1175. doi: 10.2337/diabetes.52.5.1169. [DOI] [PubMed] [Google Scholar]
- 60.Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol Endocrinol. 2006;20:1867–1879. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- 61.Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219–3227. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 62.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 63.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 64.del VCM, Reyes JM, Park CY, Gao X, Mori K, Chuck RS, Gehlbach PL. Demonstration by redox fluorometry that sulforaphane protects retinal pigment epithelial cells against oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:2606–2612. doi: 10.1167/iovs.07-0960. [DOI] [PubMed] [Google Scholar]
- 65.Hiiand CS, Howell SL. Effects of epicatechin on rat islets of Langerhans. Diabetes. 1984;33:291–296. doi: 10.2337/diab.33.3.291. [DOI] [PubMed] [Google Scholar]
- 66.Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–277. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 67.Teodoro T, Zhang L, Alexander T, Yue J, Vranic M, Volchuk A. Oleanolic acid enhances insulin secretion in pancreatic beta-cells. FEBS Lett. 2008;582:1375–1380. doi: 10.1016/j.febslet.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 68.Abdel Aziz MT, El-Asmar MF, El Nadi EG, Wassef MA, Ahmed HH, Rashed LA, Obaia EM, Sabry D, Hassouna AA, Abdel Aziz AT. The effect of curcumin on insulin release in rat-isolated pancreatic islets. Angiology. 2010;61:557–566. doi: 10.1177/0003319709356424. [DOI] [PubMed] [Google Scholar]
- 69.Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, Richard S, Quignard JF, Gross R, Petit P, Bataille D, Oiry C. Quercetin potentiates insulin secretion and protects INS-1 pancreatic beta-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161:799–814. doi: 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakab M, Lach S, Bacova Z, Langeluddecke C, Strbak V, Schmidt S, Iglseder E, Paulmichl M, Geibel J, Ritter M. Resveratrol inhibits electrical activity and insulin release from insulinoma cells by block of voltage-gated Ca+ channels and swelling-dependent Cl- currents. Cell Physiol Biochem. 2008;22:567–578. doi: 10.1159/000185541. [DOI] [PubMed] [Google Scholar]
- 72.Szkudelski T. Resveratrol inhibits insulin secretion from rat pancreatic islets. Eur J Pharmacol. 2006;552:176–181. doi: 10.1016/j.ejphar.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 73.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, Collins HW, Matschinsky FM, Stanley CA, Smith TJ. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281:10214–10221. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 74.Szkudelski T. Resveratrol-induced inhibition of insulin secretion from rat pancreatic islets: evidence for pivotal role of metabolic disturbances. Am J Physiol Endocrinol Metab. 2007;293:E901–907. doi: 10.1152/ajpendo.00564.2006. [DOI] [PubMed] [Google Scholar]
- 75.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.