Abstract
Schistosomiasis japonica is a severe tropical disease caused by the parasitic worm Schistosoma japonicum. Among the most serious pathological effects of S. japonicum infection are hepatic lesions (cirrhosis and fibrosis) and portal hypertension. Interleukin-17 (IL-17) is a pro-inflammatory cytokine involved in the pathogenesis of many inflammatory and infectious conditions, including schistosomiasis. We infected C57BL/6 mice with S. japonicum and isolated lymphocytes from the liver to identify cell subsets with high IL-17 expression and release using flow cytometry and ELISA. Expression and release of IL-17 was significantly higher in hepatic lymphocytes from infected mice compared with control mice in response to both non-specific stimulation with anti-CD3 monoclonal antibody plus/anti-CD28 monoclonal antibody and PMA plus ionomycin. We then compared IL-17 expression in three hepatic T-cell subsets, T helper, natural killer T and γδT cells, to determine the major source of IL-17 during infection. Interleukin-17 was induced in all three subsets by PMA + ionomycin, but γδT lymphocytes exhibited the largest increase in expression. We then established a mouse model to further investigate the role of IL-17 in granulomatous and fibrosing inflammation against parasite eggs. Reducing IL-17 activity using anti-IL-17A antibodies decreased infiltration of inflammatory cells and collagen deposition in the livers of infected C57BL/6 mice. The serum levels of soluble egg antigen (IL) -specific IgGs were enhanced by anti-IL-17A monoclonal antibody blockade, suggesting that IL-17 normally serves to suppress this humoral response. These findings suggest that γδT cells are the most IL-17-producing cells and that IL-17 contributes to granulomatous inflammatory and fibrosing reactions in S. japonicum-infected C57BL/6 mouse liver.
Keywords: interleukin-17, liver, natural killer T cell, Schistosoma japonicum, T helper type 17, γδT cell
Introduction
Schistosomiasis is a tropical parasitic disease caused by blood-dwelling worms of the genus Schistosoma. The main species pathogenic to humans are S. mansoni, S. japonicum and S. haematobium.1 Schistosomiasis caused by S. japonicum is endemic in China and the Philippines. Disease symptoms are due predominantly to the host immune response to schistosome eggs (ova) and the granulomatous reaction evoked.2–4 Granulomas destroy the eggs and sequester or neutralize otherwise pathogenic egg antigens but also lead to fibrogenesis in host tissues.4 In Schistosomiasis japonica, pathology develops at sites of highest egg accumulation, most often the intestines and liver. Infection by S. japonicum, a multi-cellular parasite with an extremely diverse repertoire of antigens, induces the production of multiple cytokines that mediate the immune response. These cytokines are therefore potential therapeutic targets for schistosomiasis treatment.
Lymphocytes are white blood cells of uniform appearance but varied function. Subtypes include T, B and natural killer cells. The T cells are divided into αβ T cells and γδT cells according to the subunit combination of the T-cell receptor.5 Natural killer T (NKT) cells are a small but essential subset of T lymphocytes with characteristics of both T and NK cells.6,7 These NKT and γδT cells represent approximately 20% and 1–2%, respectively, of all T lymphocytes in the liver, higher than in spleen or mesenteric lymph nodes. We took advantage of the high proportion of NKT and γδT cells in the liver to characterize the cellular sources of interleukin-17 (IL-17), a critical pro-inflammatory cytokine in the pathogenic response to S. japonicum.
Interleukin-17 activates neutrophils by inducing the release of other cytokines important in granulopoiesis (granulocyte colony-stimulating factor) and neutrophil chemotaxis (CXCL1/KC and CXCL8/IL-8).8 It is essential not only for the development of autoimmune disease but also for protection against pathogens,9 including S. japonicum.10 In fact, several studies have concluded that IL-17 is most directly associated with the severity of hepatic granulomatous inflammation.11–14 We analysed IL-17A expression by flow cytometry and IL-17 release by a specific ELISA in both infected and control mice. Previous reports concluded that IL-17 was produced mainly by T helper type 17 (Th17) cells after infection by S. japonicum.15 However, there is limited information about other lymphocyte subsets that secret IL-17 in schistosomiasis. Multiple studies have demonstrated that Th17 is not the only IL-17-producing T-cell population; CD4+ T cells, NKT cells and γδT cells are also IL-17-producing T cells in many infections, such as Listeria monocytogenes,16 Nocardia asteroides17 and Salmonella enterica serovar Typhi.18
The aim of the current study was to characterize the role of IL-17 in the pathogenic processes of the S. japonicum-infected liver. We compared IL-17 expression and secretion from three lymphocyte subsets, CD4+ T, NKT and γδT cells in response to non-specific stimulation with PMA and ionomycin and found that γδT lymphocytes were the largest IL-17-producing cell population in the mouse liver. Methods developed and validated in patients with chronic hepatic diseases have identified direct biomarkers for fibrosis, indirect multi-test batteries and physical methods to evaluate the severity of liver damage induced by S. japonicum and other pathogens.19 Among these fibrosis markers, pro-collagen type III (PC-III) and type IV collagen (IV-C) are sensitive and accurate fibrosis markers as measured by ELISA. Serum PC-III concentration reflects the difference between collagen production and elimination and is more a marker of active fibrogenesis than fibrosis.20 In this study, we also show that decreasing IL-17 with a neutralizing anti-IL-17A monoclonal antibody (mAb) increased schistosome-specific antibody levels and partially protected against S. japonicum infection in mice.
Materials and methods
Mice, parasites and infection
Female C57BL/6 mice, 6–8 weeks old, were purchased from Zhongshan University Animal Centre (Guangzhou, China) and maintained in a specific-pathogen-free facility at Guangzhou Medical College. Cercariae of S. japonicum were shed from naturally infected Oncomelania hupensis snails collected from fields in Anhui Province, China. Mice were infected percutaneously with 40 ± 5 cercariae and killed at 5–7 weeks after infection. Neutralizing rat anti-mouse IL-17A mAb or an isotype-matched rat IgG2a mAb was first administered intraperitoneally 3 weeks after S. japonicum infection (62·5 μg per mouse) then at the same dose every third day until 2 days before killing. Animal experiments were performed in strict accordance with the regulations for the Administration of Affairs Concerning Experimental Animals, and all efforts were made to minimize suffering.
Antibodies
The FITC-conjugated anti-mouse CD3 (17A2), allophycocyanin-Cy7-conjugated anti-mouse CD3 (145-2C11), Peridinin chlorophyll protein-Cy5.5-conjugated anti-mouse CD4 (RM4-5), phycoerythrin-Cy7-conjugated anti-mouse NK1.1 (PK136), FITC-conjugated anti-mouse T-cell receptor-γδCR (17A2), phycoerythrin-conjugated anti-mouse IL-17A (TC11-18H10), allophycocyanin-conjugated anti-mouse interferon-γ (IFN-γ; XMG1.2), and isotype-matched control mAb (X39, G155-178) were purchased from BD/Pharmingen (San Diego, CA). The neutralizing rat anti-mouse IL-17A mAb (clone TC11-18H10.1) and an isotype-matched rat IgG2a mAb (clone RTK2758) were purchased from BioLegend (San Diego, CA).
Isolation of lymphocytes
Mice were anaesthetized and immobilized from weeks 5 and 7 after infection. The precava was cut and sterile normal saline was injected to remove blood from the liver through the ventriculus sinister. The liver was removed, pressed through 200-gauge stainless-steel mesh, and suspended in Hanks' balanced salt solution. Lymphocytes were isolated by Ficoll–Hypaque density gradient centrifugation. Isolated cells were washed twice in Hanks' balanced salt solution and resuspended at 2 × 106 cells/ml in complete RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine and 50 μm 2-mercaptoethanol.
ELISA detection of cytokines
Single-cell suspensions were prepared and plated in 96-well micro-titre plates at 4 × 105 cells/200 μl medium per well. Anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (1 μg/ml) were added to each well and plates were incubated overnight at 4°. Supernatants were collected 72 hr later and released cytokines were measured using mouse cytokine multiplex assay kits for IFN-γ (R&D Systems Inc., Minneapolis, MN) and IL-17 (BD Pharmingen, Franklin Lakes, NJ). ELISAs were performed in accordance with the manufacturer's instructions. The optical density of each well was read at 450 nm using a microplate reader (Model ELX-800; BioTek Instruments Inc., Winooski, VT).
Detection of cell surface markers and intracellular cytokine expression
Single-cell suspensions from the livers of control mice and mice infected with S. japonicum were stimulated with 20 ng/ml PMA plus 1 μg/ml ionomycin for 5 hr at 37° under a 5% CO2 atmosphere. Brefeldin A (10 μg/ml; Sigma-Aldrich, St. Louis, MO) was added during the last 4 hr of incubation. Cells were washed twice in PBS, fixed with 4% paraformaldehyde and permeabilized overnight at 4° in PBS buffer containing 0·1% saponin (Sigma-Aldrich), 0·1% BSA and 0·05% NaN3. Cells were then stained for 30 min at 4° in the dark with conjugated antibodies specific for the cell surface antigens CD3, CD4, NK1.1, and T-cell receptor-γδ or the cytokines IFN-γ and IL-17A. The expression phenotypes of antibody-labelled lymphocytes (200 000–300 000 cells per run) were analysed by flow cytometry (BD Calibur and Aria II) and results were analysed with flowjo version 6·0 (Tree Star Inc., Ashland, OR). Isotype-matched controls for cytokines were included in each staining protocol.
Histology studies
Livers were removed from the mice, perfused three times with 0·01 m PBS (pH 7·4), fixed in 10% formalin, embedded in paraffin, and sectioned. The sections were then examined by light microscopy under 100 × and 400 × magnification after standard haematoxylin & eosin staining for visualization of cellular changes or Masson's trichrome staining for collagen deposition.
Granuloma size was measured by computer-assisted morphometric analysis using dp-2bsw software (Olympus, Shinjuku, Tokyo, Japan). Granulomas were measured under a microscope in 80−100 visual fields of liver sections (mounted on coded slides) by an observer blind to treatment history. To more accurately reflect the true shape and dimensions of the granulomas in thin sections, only cross-sections containing a visible central egg were counted. Granuloma size is expressed as mean area in μm2 ± SD.
ELISA detection of collagens
The PC-III and IV-C in serum were analysed by ELISA according to the manufacturer's instructions (BIOVALUE). Samples were read at 450 nm using a microplate reader (Model ELX-800, BioTek).
Detection of antibodies by ELISA
Immunoglobulin G and IgE antibodies to soluble egg antigen (SEA) were measured by ELISA. Briefly, SEA was dissolved in coating buffer (0·05 m sodium bicarbonate buffer, pH 9·6) at a concentration of 80 μg/ml. Then, 100 µl of this solution was added to each well and the plate was incubated overnight at 4°. After removing the solution, the plate was blocked by adding 200 μl/well blocking solution (5% skimmed milk power in 0·02 m PBS with 0·05% Tween-20, pH 7·2) at 37° for 1 hr. After the wells were emptied, 100 μl of 10-fold serially diluted serum (1 : 10 or 1 : 100 for IgE; 1 : 1000, 1 : 10 000, or 1 : 100 000 for IgG) was added to each well and incubated at 37° for 1 hr. After four washes, 100 μl horseradish peroxidase-conjugated goat anti-mouse IgG (ZB2305; ZSGB-Bio, Beijing, China) and horseradish peroxidase-conjugated goat anti-mouse IgE (40411-250; Alpha Diagnostic International, San Antonio, TX) solution diluted in PBS/Tween-20 was added and incubated at 37° for 1 hr. The plate was washed as above and the reaction was visualized by adding 100 μl TMB Substrate Reagent (BD) to each well for 10 min in the dark at room temperature. The reaction was stopped by adding 100 μl/well stop solution (1 m H2SO4) and the absorbance of each well was measured at 450 nm using an ELISA plate reader (Model ELX-800; BioTek).
Statistics
Statistical evaluation of differences between means was performed by unpaired, two-tailed Student's t-tests. P < 0·05 was considered significant.
Results
Elevated IL-17 release from cultured hepatic lymphocytes isolated from control and S. japonicum-infected mice in response to anti-CD3 mAb plus anti-CD28 mAb stimulation
Interleukin-17 may be a major contributor to schistosomiasis-associated liver pathology.13,21 To investigate whether S. japonicum infection promotes IFN-γ and IL-17 production in liver lymphocytes, we compared IFN-γ and IL-17 secretion from CD3+ T cells isolated from blood-free normal or infected liver. Release of these cytokines into the supernatant was barely detectable in cultures of unstimulated lymphocytes from normal and infected livers, but was significantly induced in both cell populations by anti-CD3 mAb plus anti-CD28 mAb stimulation. However, release was substantially higher in lymphocytes from infected livers (IFN-γ: 13·96 ± 0·37 ng/ml; IL-17: 1·14 ± 0·12 ng/ml), suggesting that the production of IFN-γ and IL-17 in the liver is markedly enhanced by infection (*P < 0·05; Fig. 1).
Figure 1.
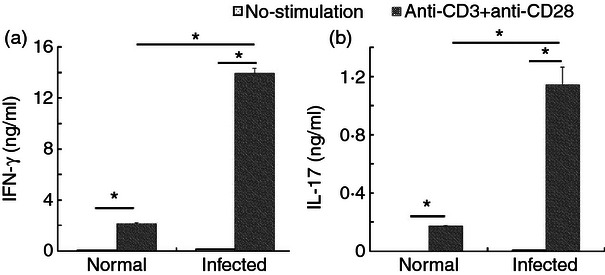
Elevated interleukin-17 (IL-17) release from cultured hepatic lymphocytes isolated from control or Schistosoma japonicum-infected mice in response to anti-CD3 monoclonal antibody (mAb) plus anti-CD28 mAb stimulation. Single liver cell suspensions of normal and infected mice were prepared and then cultured in the presence of anti-CD3 mAb plus anti-CD28 mAb. The culture supernatants were collected after 72 h of incubation for detection of interferon- γ (IFN-γ) and IL-17 by ELISA. The data are representative of four experiments, each with three or four replicates per group (*P < 0·05, the error bars indicate SD).
The proportion of IL-17+ cells in the total T-cell population isolated from infected and uninfected liver
Interleukin-17 expression by lymphocytes is a marker of disease severity in schistosomiasis.22 To estimate the IFN-γ and IL-17A release capacity of CD3+ cells isolated from blood-free normal and infected C57BL/6 mouse livers, expression of both cytokines in response to PMA + ionomycin stimulation was measured by FACS (Fig. 2a). These experiments revealed significantly higher percentages of IL-17+ CD3+ and IFN-γ+ CD3+ cells in the lymphocyte population isolated from infected livers compared with uninfected livers following stimulation (IFN-γ: 17·02 ± 6·22% versus 4·11 ± 2·75%, P < 0·05, Fig. 2b; IL-17: 4·03 ± 1·27% versus 2·02 ± 0·85%, P < 0·01, Fig. 2c). Hence, consistent with IFN-γ and IL-17 release (Fig. 1), a significantly higher percentage of T cells from infected livers expressed these cytokines following activation. Moreover, in parallel with the result obtained with CD3+ cells, the proportion of IL-17+ cells and IFN-γ+ cells in CD3− cells increased after infection (IFN-γ: 6·95 ± 5·11% versus 2·00 ± 1·58%, P < 0·05, Fig. 2b; IL-17: 1·49 ± 0·85% versus 0·35 ± 0·17%, P < 0·01, Fig. 2c). Additionally, the proportions of both IL-17+ CD3+ and IFN-γ+ CD3+ cells were significantly higher than of CD3− cells in both normal control and infected group (P < 0·05).
Figure 2.
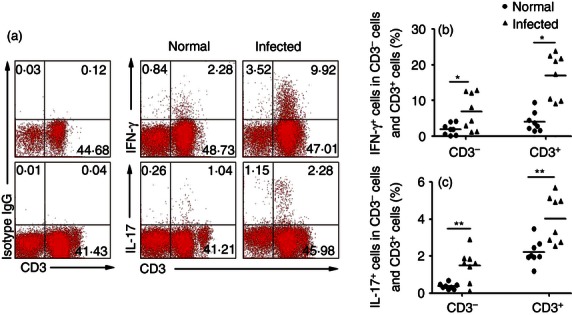
The proportion of interleukin-17-positive (IL-17+) cells in the CD3− cells and CD3+ cell population isolated from control or infected liver. Six weeks after the infection, the mice were killed. Single cell suspensions of liver cells were stimulated with PMA, ionomycin and brefeldin A. Cells were stained with anti-CD3-FITC and then intracellularly stained with phycoerythrin-conjugated antibodies against IL-17 and allophycocyanin-conjugated anti-mouse interferon-γ (IFN-γ) and also isotype IgG2a control antibody. (a) The IFN-γ and IL-17 expressions of CD3− cells and CD3+ cells from control or infected liver were analysed by flow cytometry. Flow cytometric analysis from one representative experiment. (b, c) Average percentages of IFN-γ+ T cells and IL-17+ T-cell populations in normal and infected mice were calculated from FACS data (*P < 0·05).
IL-17 expression in specific T-cell subsets isolated from infected and uninfected mouse liver
Interleukin-17-producing T cells, including γδT cells, NKT cells and CD4+ Th cells, have been implicated in the development of granulomatous disease.23–25 Therefore, we investigated whether expression of IL-17 was elevated in γδT cells, NKT cells, and CD4+ helper T cells during S. japonicum infection. Lymphocytes were isolated from livers of control and infected C57BL/6 mice, counted, stimulated by PMA + ionomycin, and IL-17 expression detected by FACS. The IL-17+ fraction was higher in each subtype from S. japonicum-infected mice (Fig. 3a–c), with γδT cells showing the highest percentage (Fig. 3c) and the highest MFI of IL-17+ cells (Fig. 3d). The proportion of IL-17+ cells in the uninfected γδT-cell population was 9·11 ± 6·39% after stimulation and 25·40 ± 4·76% in the infected γδT-cell population (Fig. 3c, P < 0·01). The percentage of IL-17+ cells in the γδT cells was significantly higher than that CD4+ T cells (3·28%±1·83%) and NKT cells (2·72 ± 1·38%) after S. japonicum infection (Fig. 3c, **P < 0·01). There was no significant difference between the proportion of IL-17+ γδT cells (0·89 ± 0·38%) and IL-17+ CD4+ T (1·05 ± 0·64%) cells in the total infected and stimulated T-cell population, whereas they were both higher than infected/stimulated IL-17+ NKT cells (0·49 ± 0·31%, Fig. 3b, P < 0·05). γδT lymphocytes also exhibited the highest geometric mean fluorescence intensity (MFI) of the three subsets (γδT cells: 206·24 ± 45·81; CD4+ T cells: 108·22 ± 21·10; NKT cells: 62·09 ± 15·78; γδT versus CD4+ T cells, P < 0·05; γδT versus NKT cells, P < 0·01; CD4+T versus NKT cells, P < 0·05.). Hence, we conclude that all three subpopulations of T lymphocytes in infected liver are sources of IL-17 after non-specific stimulation, but that γδT lymphocytes produce the most IL-17.
Figure 3.
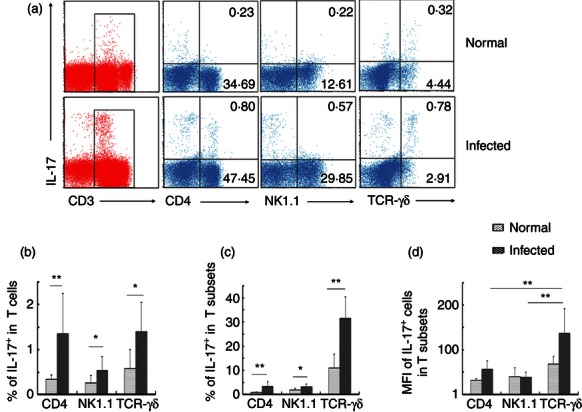
Interleukin-17 (IL-17) expression in specific T-cell subsets isolated from control or infected mouse liver. Female C57BL/6 mice were infected with 40 ± 5 Schistosoma japonicum cercariae per mouse. Six weeks after the infection, the mice were killed. Single-cell suspensions of liver cells were stimulated with PMA, ionomycin and brefeldin A. Cells were stained with anti-CD3-allophycocyanin-Cy7, anti-T-cell receptor-γδ-FITC, anti-CD4-Peridinin chlorophyll protein-Cy5.5, anti-NK1.1-phycoerythrin-Cy7 and then intracellularly stained with anti-IL-17A-phycoerythrin for FACS analysis. (a) Intracellular IL-17 expression by gated populations of CD4+ T cells, natural killer T (NKT) cells and γδT cells isolated from normal and infected mice, respectively. The data are representative of eight experiments giving similar results. (b) Percentage of the IL-17+ NK1.1+, IL-17+ γδTCR+, IL-17+ CD4+ cells in CD3+ T cells were calculated from eight independent experiments with similar results. (c) The percentage of IL-17+ cells in CD4+ T cells, NKT cells and γδT cells were shown. (d) The percentage of mean fluorescence intensity of IL-17+ cells in CD4+ T cells, NKT cells and γδT cells (*P < 0·05, **P < 0·01, the error bars indicate SD).
Reduction of hepatic granulomatous inflammation by anti-IL-17 mAb in vivo
Control, infected and infected/anti-IL-17 mAb-treated mice were killed and the morphological and histopathological differences in livers were evaluated. Normal liver was light red with a smooth surface following blood removal (Fig. 4a), whereas the infected liver was darker red with many small white spots on the surface, indicating severe inflammation and many pyogenic granulomas. In contrast, livers from infected/anti-IL-17 mAb-treated mice resembled control livers, with few small white spots, indicating reduced inflammation and granuloma response (Fig. 4a). Haematoxylin and eosin staining of liver sections revealed the normal cellular organization of uninfected hepatic lobules, with the typical actinomorphous distribution of hepatic cord centred around central veins. Infection by S. japonicum markedly altered the histological structure of the mouse liver. There were many more inflammatory cells in the infected liver lobules than in uninfected controls, while anti-IL-17A neutralizing mAb markedly reduced numbers of inflammatory cells in infected liver (Fig. 4b). The extent of hepatic granulomatous inflammation around schistosome eggs was measured by computer-assisted morphometric analysis. Infected livers developed large liver granulomas (mean cross-sectional area, 10·95 ± 0·31 μm2 × 103), whereas these granulomas were significantly smaller in infected/anti-IL-17 mAb-treated mice (6·18 ± 2·09 × 103 μm2; Fig. 4c; P < 0·05). Taken together, these results suggest that generation of IL-17 during S. japonicum infection may enhance hepatic granulomatous inflammation, consistent with previous studies implicating IL-17 in hepatic immunopathology associated with schistosomiasis.
Figure 4.
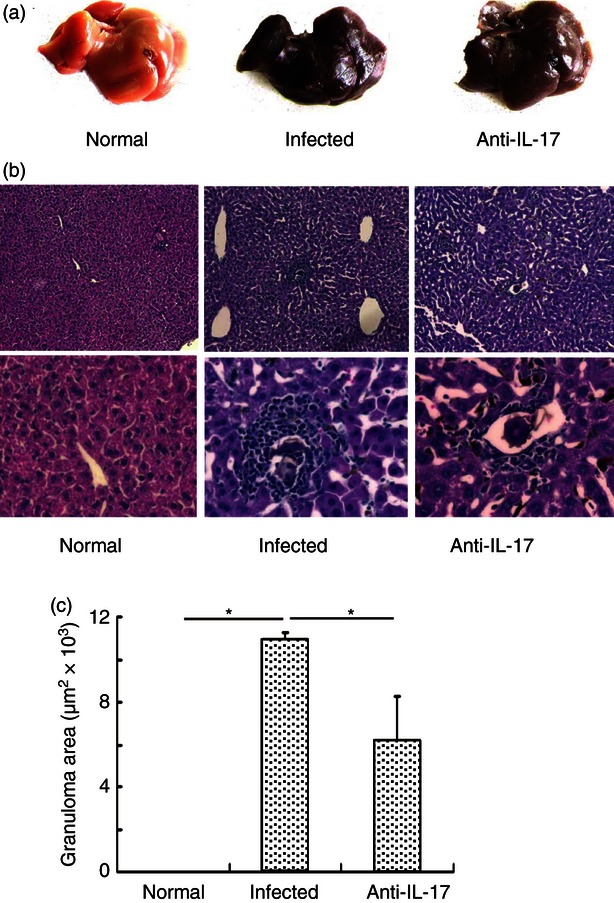
Reduction of hepatic granulomatous inflammation by anti-interleukin-17 (IL-17) monoclonal antibody (mAb) in vivo. Thirty female C57BL/6 mice were divided into three groups, normal group, infected group, anti-mouse IL-17 group. The infected group and anti-mouse IL-17 group were infected with 40 ± 5 cercariae of Schistosoma japonicum per mouse. For the infected and anti-IL-17 group, 62·5 μg control IgG mAb or anti-IL-17 mAb per mouse were administered intraperitoneally every 3 days, a total of four times, respectively. Six weeks after the infection, the mice were killed. (a) The gross appearance of the three groups. (b) Livers were flushed with 0·01 m PBS three times, fixed in 10% formalin, embedded in paraffin and sectioned. Sections of the liver of normal mice (left panels), infected mice (middle panels) and anti-mouse IL-17 mAb mice (right panels) were examined by haematoxylin & eosin staining (original magnification × 100 for upper panels and × 400 for lower panels). In the control IgG mAb group and anti-IL-17 mAb group, granuloma could be observed. (c) Sizes of the granulomas were measured by computer-assisted morphometric analysis. Only granulomas with a visible central egg were analysed for accuracy. Between 80 and 100 granulomas for each group were measured under a microscope (*P < 0·05, the error bars indicate SD).
Reduction of hepatic fibrosing inflammation by anti-IL-17 mAb in vivo
Granulomatous and fibrosing inflammation against parasite eggs are the pathological hallmarks of schistosome infection.13 To measure interstitial fibrosis, liver tissues were cut into 4-mm sections and stained with Masson's trichrome, which stains collagen deposits blue. Masson's trichrome-positive fibrosis (indicative of collagen deposition) and infiltration of inflammatory cells were significantly elevated in infected livers compared with uninfected control and infected/anti-IL-17A mAb-treated livers (Fig. 5a). Serum levels of PC-III and IV-C were detected by ELISA (Fig. 5a,b). Serum levels of PC-III were significantly higher in infected mice compared with both uninfected control and infected/anti-IL-17 mAb-treated mice (Infected: 19·31 ± 3·63 ng/ml; Control: 13·86 ± 1·28 ng/ml; Anti-IL-17: 12·10 ± 3·43 ng/ml; P < 0·05 for both control and anti-IL-17A mAb-treated livers compared with infected livers, Fig. 5b). In contrast, IV-C levels were significantly higher in uninfected control mice than in both infected and infected/anti-IL-17 mAb-treated mice (Control: 15·24 ± 0·87 ng/ml; Infected: 12·90 ± 1·44 ng/mL; Anti-IL-17: 12·83 ± 1·56 ng/ml; P < 0·05 for both treatment groups compared with control mice; Fig. 5c), whereas there was no significant difference in IV-C levels between infected and infected/anti-IL-17 mAb-treated mice (Fig. 5c). Hence, IL-17 moderately decreased IV-C expression during S. japonicum infection. Interleukin-17 appears to differentially regulate the expression of collagen isoforms and contributes to hepatic fibrosis by promoting PC-III synthesis and deposition.
Figure 5.
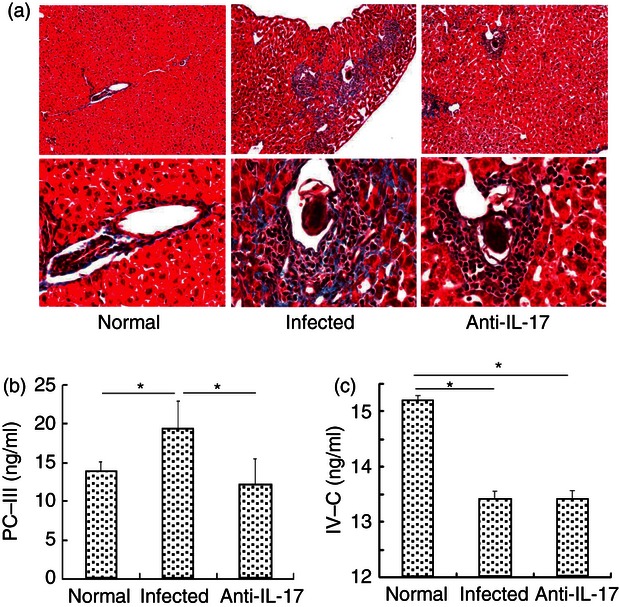
Reduction of hepatic fibrosing inflammation by anti-interleukin-17 (IL-17) monoclonal antibody (mAb) in vivo. Female C57BL/6 mice were divided into three groups, normal group, infected group and anti-mouse IL-17 mAb group. The infected and anti-IL-17 mAb groups were infected with 40 ± 5 cercariae of Schistosoma japonicum per mouse. For infected and group, 62·5 μg of control IgG mAb or anti-IL-17 mAb per mouse were administered intraperitoneally every three days, for a total of four times, respectively. Six weeks after the infection, the mice were killed. (a) Livers were flushed with 0·01 m PBS three times, fixed in 10% formalin, embedded in paraffin, and sectioned. Sections of the liver of normal, control IgG mAb and anti-IL-17 mAb mice were examined by Masson's trichrome staining (× 100 for upper panels, × 400 for lower panels). (b and c) Levels of pro-collagen type III (PC-III) and type IV collagen (IV-C) in the serum of three groups were detected by ELISA (*P < 0·05, the error bars indicate SD).
Role of IL-17 in SEA-specific IgG and IgE production
The evolving cellular response to S. japonicum infection is gradually accompanied by production of non-complement fixing IgG and IgE antibodies.26 There were significant differences in the serum levels of SEA-specific IgG and IgE antibodies between uninfected control mice, mice infected with S. japonicum, and infected mice treated with anti-IL-17A mAb as detected by ELISA. Infected mice exhibited significantly elevated SEA-specific IgG (Fig. 6a; P < 0·05) and IgE (Fig. 6b; P < 0·05) levels compared with control mice. Levels of SEA-specific IgG were even higher in the anti-IL-17 group, although not significantly, while IgE levels were not altered by anti-IL-17A mAb. These effects held across different serum dilutions, suggesting that there was no saturation of ELISA binding sites. Hence, IL-17 normally serves to suppress SEA-specific IgG expression, but has little effect on SEA-specific IgE expression.
Figure 6.
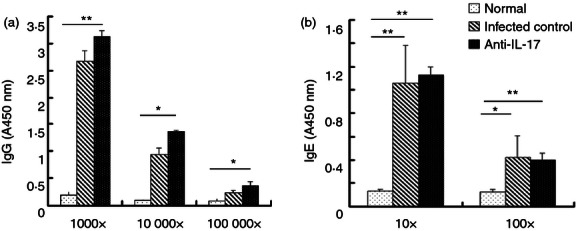
Role of interleukin-17 monoclonal antibody (IL-17 mAb) in soluble egg antigen (SEA) -specific IgG and IgE production. SEA-specific IgG and IgE antibodies in serum from normal, infected and anti-IL-17 mAb treated mice were detected by ELISA. All the samples were collected from 6-week-infected mice. The sera were serially diluted (1 : 10 or 1 : 100 for IgE; 1 : 1000, 1 : 10 000, or 1 :100 000 for IgG). IgG and IgE levels are expressed as the A450 values of the individual sera (*P < 0·05, **P < 0·01, the error bars indicate SD).
Discussion
Interleukin-17 is a pro-inflammatory cytokine that facilitates mucosal neutrophil recruitment by inducing the expression of downstream cytokines that mediate granulopoiesis as well as activating the local production of CXCR2 ligands that are chemoattractants for neutrophils.27 It is a major inflammatory cytokine during infection, including infection by S. japonicum.9,21,28 Schistosomes are parasitic trematodes that infect and cause disease in many vertebrate species. The main adaptive immune response against schistosomes is mediated by MHC class II-restricted CD4+ T cells. An initial pro-inflammatory Th1-polarized response lasts into the period of early oviposition at around 5 weeks post-infection, at which time peri-oval granulomatous inflammation begins. However, within the next 1–2 weeks, granuloma formation increases concomitant with a dramatic change in the cytokine environment, which under normal circumstances becomes dominated by anti-inflammatory Th2-type cytokines.26 Interleukin-4, IL-5 and IL-13 have previously been implicated in S. japonicum-associated liver fibrosis in mice and in humans.29–32 Moreover, the granulomas that formed around the eggs in the liver were reported to be positively regulated by IL-17.13,21,22
We measured IL-17 production from mononuclear cells isolated from healthy and infected mouse livers by ELISA and FACS. Stimulation by anti-CD3 mAb plus anti-CD28 mAb induced much greater IL-17 release (and IFN-γ release) from lymphocytes of infected mice than from lymphocytes of uninfected mice (P < 0·05) (Fig. 1). Moreover, non-specific stimulation by PMA and ionomycin induced a much greater increase in the proportion of IL-17+ cells in the CD3+ T-cell population from infected livers than in the CD3+ T-cell population from uninfected livers (Fig. 2). Hence, secretion of IL-17 by liver lymphocytes was significantly enhanced by S. japonicum infection.
The normal liver contains significant numbers of resident lymphocytes, including T cells, B cells, dendritic cells, and natural killer cells. Recent reports have suggested that IL-17 is produced by natural killer cells, NKT cells, γδT cells, neutrophils and CD8+ T cells in addition to CD4+ T (Th17) cells, at least in some diseases.23–25 Our results (Fig. 2) demonstrated that S. japonicum infection could induce a small fraction of CD3− lymphocytes to produce IL-17, too. The CD3− lymphocytes may include B cells, dendritic cells, natural killer cells and neutrophils, so further investigation is required.
Previous reports indicated that CD4+ T cells are the dominant IL-17 producers.33,34 Moreover, Xiaoyun Wen et al. reported that the proportion of Th17 cells in the spleen, mesenteric lymph nodes and liver increased during S. japonicum infection, whereas Yuxia Zhang et al. reported that CD4+ T (Th17) cells isolated from the hepatic granulomatous cell clusters of mice 6 weeks post-infection are the major IL-17-producing cells.15,34 However, γδT cells are thought to be the main source of IL-17 in several infectious conditions.35–37 Studies of infected mice also found that the number of IL-17-producing γδT cells far exceeded the number of IL-17-producing CD4+ T cells.38,39 In the current study, we compared IL-17 expression in CD4+ T, NKT and γδT lymphocyte subsets from normal liver and infected liver in response to non-specific stimulation with PMA and ionomycin. As shown in Fig. 3, NKT cells and γδT cells were also sources of IL-17, and the proportions of IL-17+ CD4, NKT and γδT cells were significantly higher in infected mice than in uninfected control mice (P < 0·05). The IL-17+ CD4+ T and IL-17+ γδT fractions accounted for 1·05 ± 0·64% and 0·89 ± 0·38% of the total CD3+ T population after infection, respectively, both significantly higher than the IL-17+ NKT fraction (0·49 ± 0·31%). However, IL-17+ γδT cells emitted the highest immunofluorescence of the three T-cell subtypes. We conclude that all three cell types are sources of IL-17 during S. japonicum infection, but that of these cell subtypes, γδT cells produced the most IL-17. These results indicate that γδT cells may be a first line of defence inflammation before CD4+ T-cell responses during S. japonicum infection. These γδT cells are a unique T-cell population because they function as part of the innate immune response and are capable of responding to cytokine signals in the absence of antigen,40 demonstrating antigen specificity but requiring neither priming nor recruitment to the site of infection. Further investigations are required to elucidate the full spectrum of effects of this cell type during infection.
Infection with S. japonicum results in a granulomatous inflammatory and fibrosing reaction in liver and intestine against parasite eggs.22 Granuloma formation is the result of a host adaptive immune response mediated by CD4+ T cells specific for SEAs3,22,26 that damages hepatocytes and destroys the normal histological structure of the liver (Fig. 4a). The main pathological lesions of hepatic schistosomiasis are the granuloma formed around schistosome eggs at the acute stage of the infection and subsequent liver fibrosis at chronic and advanced stages.41 Hepatic fibrosis during schistosomiasis results from a massive deposition of extracellular matrix in the periportal spaces, leading to blockage of the portal veins with ensuing portal hypertension, as well as splenomegaly, portocaval shunting and gastrointestinal varices.2 Persistent fibrosis in chronic schistosomiasis may cause hepatic cirrhosis and liver cancer, both associated with high mortality. The severity of liver pathogenesis may be correlated with IL-17 activity, possibly released by inflammatory cells such as neutrophils and eosinophils in the granulomas.22 We administered an anti-IL-17 mAb to infected mice to evaluate the role of IL-17 in the host protective responses against S. japonicum infection. Gross examination of livers excised from control, infected and infected/anti-IL-17 mAb-treated mice (Fig. 4a) indicated that infection caused inflammatory cell infiltration that was partially blocked by anti-IL-17 mAb, strongly implicating IL-17 in this response. Moreover, schistosome infection also produced hepatic granulomatous inflammation, which was significantly reduced by anti-IL-17 mAb (P < 0·05 compared with infected mice) as measured by granuloma cross-sectional area. Taken together, these results are consistent with previous reports demonstrating a positive relationship between IL-17 activity and the severity of liver pathogenesis and reports implicating IL-17 in the granulomatous inflammatory reaction during S. japonicum infection.
Histological observations using Masson's trichrome staining also revealed liver fibrosis (collagen deposition) and inflammatory cell infiltration in infected mouse liver, responses that were also suppressed by anti-IL-17 mAb (Fig. 5a). Both pro-peptide and mature collagen type III can be used as a biomarker for liver fibrosis, and serum levels of type IV collagen predict the state of liver fibrosis,42 which in turn reflects collagen degradation.43 Hence, the levels of PC-III and IV-C may reflect the current pathological progress of schistomiasis.44 Comparison of infected and infected/anti-IL-17 mAb-treated mice suggested that IL-17 may increase PC-III expression, possibly by inducing type III collagen synthesis, whereas anti-IL-17 mAb may attenuate fibrosis by reducing extracellular collagen III deposition in the liver (Fig 5b). In contrast to PC-III, the difference in hepatic IV-C expression between infected and infected/anti-IL-17 mAb-treated mice was minimal (Fig 5c), suggesting that IL-17 does not regulate collagen degradation. An alternative hypothesis is that the level of IV-C may be affected by other pathological conditions, such as lung or intestine failure, but this requires further investigation. Taken together, these data demonstrated that IL-17 contributes to the formation and development of fibrosis during S. japonicum infection and that targeting IL-17 may have therapeutic potential for treatment of schistosomiasis.
Numerous in vitro studies on the immunology of schistosomiasis have clearly demonstrated the essential role of antibodies in various effector or regulatory mechanisms.45 Abundant productions of non-complement fixing IgG and IgE antibodies were measured after infection. In our study, the levels of SEA-specific IgG and IgE antibodies in mouse serum were measured to examine associations between schistosome-specific antibody responses and anti-IL-17 mAb treatment during S. japonicum infection. The serum titres of SEA-specific IgGs and IgEs increased rapidly during infection. The level of SEA-specific IgG was actually increased further by anti-IL-17 mAb, whereas there was no significant effect of this treatment on IgE titres, which is consistent with the results of Xiaoyun Wen et al.15 As the humoral response plays an important role in anti-schistosome immunity,46,47 IL-17 may be a target for therapeutic intervention in schistosomiasis patients, as it is in inflammatory bowel disease and mycoplasma pneumonia.48,49
In conclusion, we demonstrate that the IL-17+ lymphocyte population expands significantly in the liver of C57BL/6 mice during infection by S. japonicum. In particular, γδT lymphocytes represented the largest population of IL-17-producing cells. Moreover, we found that IL-17 was involved in granuloma formation, infiltration of inflammatory cells and collagen deposition. Anti-IL-17 mAb therapy may be helpful in preventing inflammation-mediated liver damage caused by schistosomiasis.
Acknowledgments
This work was supported by a grant from the Natural Science Foundation of China (30901353), Natural Science Foundation of Hunan Province (11JJ3103) and Science and Technology Planning Project of Guangzhou City (2011J22007). The authors thank Prof. Wu Changyou in the Institute of Immunology at Zhongshan School of Medicine, Sun Yat-sen University provide help in intracellular cytokines staining.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–97. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–54. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauritsen JP, Haks MC, Lefebvre JM, Kappes DJ, Wiest DL. Recent insights into the signals that control αβ/γδ-lineage fate. Immunol Rev. 2006;209:176–90. doi: 10.1111/j.0105-2896.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 7.Werner JM, Busl E, Farkas SA, Schlitt HJ, Geissler EK, Hornung M. DX5+ NKT cells display phenotypical and functional differences between spleen and liver as well as NK1.1-BALB/c and NK1.1+ C57BL/6 mice. BMC Immunol. 2011;12:26. doi: 10.1186/1471-2172-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Ghani RA, Hassan AA. Murine schistosomiasis as a model for human Schistosoma mansoni: similarities and discrepancies. Parasitol Res. 2010;107:1–8. doi: 10.1007/s00436-010-1855-5. [DOI] [PubMed] [Google Scholar]
- 12.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–76. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 13.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–95. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 14.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–67. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen X, He L, Chi Y, et al. Dynamics of Th17 cells and their role in Schistosoma japonicum infection in C57BL/6 mice. PLoS Negl Trop Dis. 2011;5:e1399. doi: 10.1371/journal.pntd.0001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada S, Umemura M, Shiono T, et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–63. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam S, Maksaereekul S, Hyde DM, Godinez I, Beaman BL. IL-17 and γδ T-lymphocytes play a critical role in innate immunity against Nocardia asteroides GUH-2. Microbes Infect. 2012;14:1133–43. doi: 10.1016/j.micinf.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McArthur MA, Sztein MB. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS ONE. 2012;7:e38408. doi: 10.1371/journal.pone.0038408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670–81. doi: 10.1053/j.gastro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Zachariae H, Heickendorff L, Sogaard H. The value of amino-terminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol. 2001;144:100–3. doi: 10.1046/j.1365-2133.2001.03959.x. [DOI] [PubMed] [Google Scholar]
- 21.Rutitzky LI, Lopes DRJ, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–6. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 22.Smith PM, Shainheit MG, Bazzone LE, Rutitzky LI, Poltorak A, Stadecker MJ. Genetic control of severe egg-induced immunopathology and IL-17 production in murine schistosomiasis. J Immunol. 2009;183:3317–23. doi: 10.4049/jimmunol.0901504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 24.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 25.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 26.Gause WC, Urban JJ, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 27.Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med. 2008;8:416–26. doi: 10.2174/156652408785160998. [DOI] [PubMed] [Google Scholar]
- 28.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 29.Qi Y, Song XR, Shen JL, et al. Tim-2 up-regulation and galectin-9-Tim-3 pathway activation in Th2-biased response in Schistosoma japonicum infection in mice. Immunol Lett. 2012;144:60–6. doi: 10.1016/j.imlet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Seki T, Kumagai T, Kwansa-Bentum B, Furushima-Shimogawara R, Anyan WK, Miyazawa Y, Iwakura Y, Ohta N. Interleukin-4 (IL-4) and IL-13 suppress excessive neutrophil infiltration and hepatocyte damage during acute murine schistosomiasis japonica. Infect Immun. 2012;80:159–68. doi: 10.1128/IAI.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutinho HM, Acosta LP, Wu HW, et al. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195:288–95. doi: 10.1086/510313. [DOI] [PubMed] [Google Scholar]
- 32.Xie H, Chen D, Luo X, Gao Z, Fang H, Huang J. Some characteristics of IL-5-producing T cells in mouse liver induced by Schistosoma japonicum infection. Parasitol Res. 2013;112:1945–51. doi: 10.1007/s00436-013-3350-2. [DOI] [PubMed] [Google Scholar]
- 33.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Chen L, Gao W, et al. IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur J Immunol. 2012;42:1523–35. doi: 10.1002/eji.201141933. [DOI] [PubMed] [Google Scholar]
- 35.Godinez I, Raffatellu M, Chu H, et al. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009;77:387–98. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber M, Heink S, Grothe H, et al. A Th17-like developmental process leads to CD8+ Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–25. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 38.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–83. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 40.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Liang YJ, Luo J, Yuan Q, et al. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS ONE. 2011;6:e20247. doi: 10.1371/journal.pone.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–17. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukamoto T, Yamamoto T, Ikebe T, Takemura S, Shuto T, Kubo S, Hirohashi K, Kinoshita H. Serum markers of liver fibrosis and histologic severity of fibrosis in resected liver. Hepatogastroenterology. 2004;51:777–80. [PubMed] [Google Scholar]
- 44.Nara T, Iizumi K, Ohmae H, et al. Antibody isotype responses to paramyosin, a vaccine candidate for schistosomiasis, and their correlations with resistance and fibrosis in patients infected with Schistosoma japonicum in Leyte, The Philippines. Am J Trop Med Hyg. 2007;76:384–91. [PubMed] [Google Scholar]
- 45.Vereecken K, Naus CW, Polman K, Scott JT, Diop M, Gryseels B, Kestens L. Associations between specific antibody responses and resistance to reinfection in a Senegalese population recently exposed to Schistosoma mansoni. Trop Med Int Health. 2007;12:431–44. doi: 10.1111/j.1365-3156.2006.01805.x. [DOI] [PubMed] [Google Scholar]
- 46.Noya O, Fermin Z, Alarcon DNB, Losada S, Colmenares C, Hermoso T. Humoral immune response of children with chronic schistosomiasis. Isotype recognition of adult worm antigens. Parasite Immunol. 1995;17:319–28. doi: 10.1111/j.1365-3024.1995.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 47.Harn DA, Danko K, Quinn JJ, Stadecker MJ. Schistosoma mansoni: the host immune response to egg antigens. I. Partial characterization of cellular and humoral responses to pI fractions of soluble egg antigens. J Immunol. 1989;142:2061–6. [PubMed] [Google Scholar]
- 48.Kurai D, Nakagaki K, Wada H, et al. Mycoplasma pneumoniae extract induces an IL-17-associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation. 2013;36:285–93. doi: 10.1007/s10753-012-9545-3. [DOI] [PubMed] [Google Scholar]
- 49.Jin Y, Lin Y, Lin L, Zheng C. IL-17/IFN-γ interactions regulate intestinal inflammation in TNBS-induced acute colitis. J Interferon Cytokine Res. 2012;32:548–56. doi: 10.1089/jir.2012.0030. [DOI] [PubMed] [Google Scholar]


