Abstract
All-solid-state secondary batteries that employ inorganic solid electrolytes are desirable because they are potentially safer than conventional batteries. The ionic conductivities of solid electrolytes are currently attracting great attention. In addition to the conductivity, the mechanical properties of solid electrolytes are important for improving the energy density and cycle performance. However, the mechanical properties of sulfide electrolytes have not been clarified in detail. Here, we demonstrate the unique mechanical properties of sulfide electrolytes. Sulfide electrolytes show room temperature pressure sintering. Ionic materials with low bond energies and a highly covalent character, which is promising for achieving a high ionic conductivity, tend to be suitable for room-temperature processing. The Young's moduli of sulfide electrolytes were measured to be about 20 GPa, which is an intermediate value between those of typical oxides and organic polymers.
There is a strong demand to develop next-generation battery systems1,2. All-solid-state secondary batteries are desirable because they are potentially safer than conventional batteries. Solid electrolytes are a critical component of all-solid-state batteries. Much effort has gone into finding new solid electrolytes with high ionic conductivities. New solid electrolytes such as Li3P7S11, Li10GeP2S12, and Na3PS4 have recently reported3,4,5,6 that exhibit high ionic conductivities; some have conductivities close to those of liquid electrolytes.
Up until recently, the most pressing challenge has been to improve the electrical and electrochemical properties of solid electrolytes3,4,5. However, although the mechanical properties of solid electrolytes (such as elastic modulus and densification behavior) are also critical for the practical development of all-solid-state batteries, few studies have investigated the mechanical properties of high-ion-conducting solid electrolytes.
In lithium secondary batteries, the volume of the electrode active material changes during charging and discharging. This results in fragmentation of electrode particles, which is one cause of capacity fading of batteries. The elastic modulus of solid electrolytes affects this fragmentation of electrode materials: specifically, solid electrolytes should have a moderate elastic modulus that is not too high or too low.
There have been few reports regarding the mechanical properties of sulfide solid electrolytes due to the difficulty in measuring them. Since most sulfide solid electrolytes are unstable in air, they have to be handled in an inert atmosphere. In a recent paper6, we reported that sulfide solid electrolytes exhibit unique mechanical properties, namely the electrolytes show a high ionic conductivity only after pressing at room temperature. Therefore, all-solid-state cells which are constructed by pressing at room temperature function as secondary batteries. However, the origin of this densification of sulfide electrolytes has not been clarified.
We have been investigating the unique behavior of sulfide solid electrolytes. In this study, we determine the mechanical properties of sulfide solid electrolytes, such as their densification under high pressure and Young's modulus. Densification is investigated by performing compression tests on solid electrolyte powder and cross-sectional SEM observations of compressed pellets. The Young's moduli of glassy solid electrolytes are determined by the ultrasonic pulse method. Furthermore, we demonstrate composite electrodes with an effective solid electrode/solid electrolyte interface designed with sulfide solid electrolytes having favorable mechanical properties.
Results
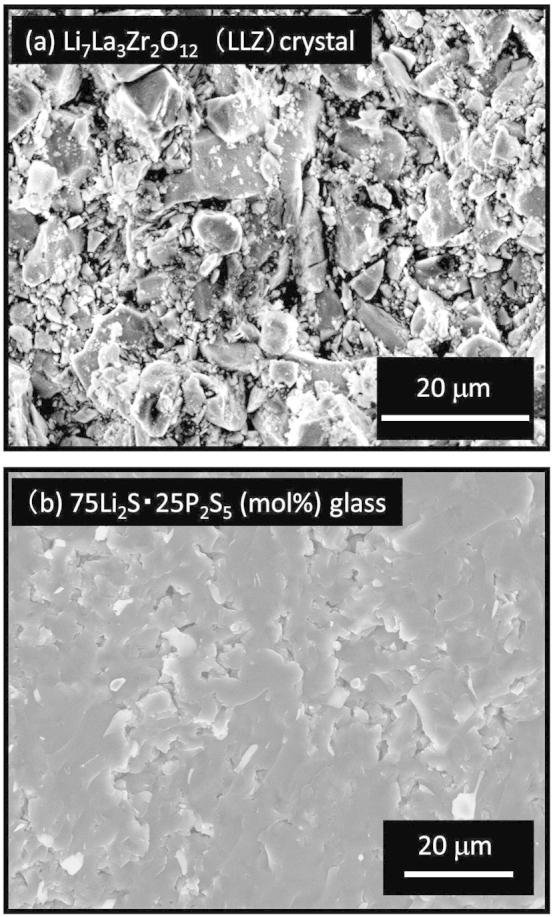
Dense pellets of sulfide electrolytes can be obtained by pressing at room temperature. Figure 1 shows scanning electron microscopy (SEM) images of fracture cross sections of typical solid oxide (Li7La3Zr2O127) and sulfide electrolyte particles (80Li2S·20P2S5 (mol.%) glass8). The samples were prepared from their respective solid electrolyte particles by pressing at 360 MPa at room temperature (cold pressing). The cross-sectional SEM images reveal Li7La3Zr2O12 particles with similar sizes to those prior to pressing. As is well known, oxide ceramics such as Li7La3Zr2O12 need to be sintered at high temperatures of over 1000°C to prepare dense samples7. In contrast, densification is induced in sulfide Li2S–P2S5 glasses just by cold pressing.
Figure 1. SEM images of fracture cross sections of typical solid oxide electrolyte Li7La3Zr2O12 and solid sulfide electrolyte particles (80Li2S·20P2S5 (mol.%)) glass pelletized at room temperature.
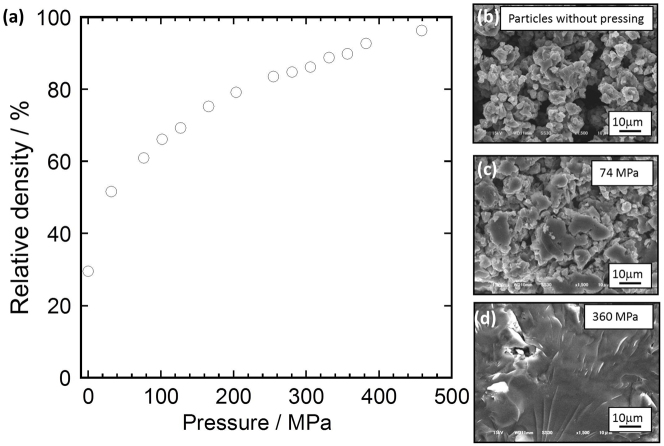
This densification is investigated by performing compression tests. Glassy solid electrolytes in the system Li2S–P2S5 are selected as typical sulfide solid electrolytes. These solid electrolytes are composed of lithium (Li+) and thiophosphate ions (PS43–). Figure 2a shows the dependence of the relative density of the 75Li2S·25P2S5 (mol.%) glass solid electrolytes on the molding pressure. In this measurement, the solid electrolyte (500 mg) was uniaxially compressed in a 10-mm-diameter mold. The total relative density was determined using 75Li2S·25P2S5 transparent glass prepared by hot pressing at about 200°C (see Fig. 3b and Ref. 9); the density of the glass was 1.88 g cm−3. The relative density increases with increasing applied pressure. It exceeds 90% when the 75Li2S·25P2S5 glass powder is compressed by a pressure of over 350 MPa. Thus, 75Li2S·25P2S5 glass with a remarkably high relative density can be obtained by pressing without heat treatment. Figures 2b–d show SEM images of 75Li2S·25P2S5 glass powder before pressing (Fig. 2b) and fracture cross sections of 75Li2S·25P2S5 glass powder compressed at 74 MPa (Fig. 2c) and 360 MPa (Fig. 2d). The particles in the glass powder are several to ten micrometers in size. The particle size of the 75Li2S·25P2S5 glass increased on applying a higher pressure. Figure 3a shows an SEM image of the cross section of the 80Li2S·20P2S5 glassy solid electrolyte compressed at 360 MPa at 25°C. The smooth cross section was prepared by ion milling. The SEM image shows a dense cross section. Grain boundaries are hardly visible, indicating that the particles grow together, increasing the particle size. The void volume is very small, which is consistent with the above-mentioned compression test results. The local environment of the sulfide ions was measured by Raman spectroscopy and found to be nearly unaltered by pressing (Supplementary Fig. S1). In addition, the XRD patterns of the glasses almost unchanged before and after pressing (Supplementary Fig. S2). Fig. 3b shows an SEM image of the solid electrolyte hot pressed at 200°C. Since this temperature is close to the glass transition temperature, dense pellets with small void volumes (they are thus transparent) were obtained by hot pressing9.
Figure 2.
(a) Molding pressure dependence of relative density of 75Li2S·25P2S5 (mol.%) glass solid electrolyte. (b) SEM image of 75Li2S·25P2S5 glass powder. SEM images of fracture cross sections of 75Li2S·25P2S5 glass powders compressed at (c) 74 and (d) 360 MPa.
Figure 3. SEM images of cross sections of 80Li2S·20P2S5 glassy solid electrolytes compressed at 360 MPa at (a) 25 and (b) 200°C.
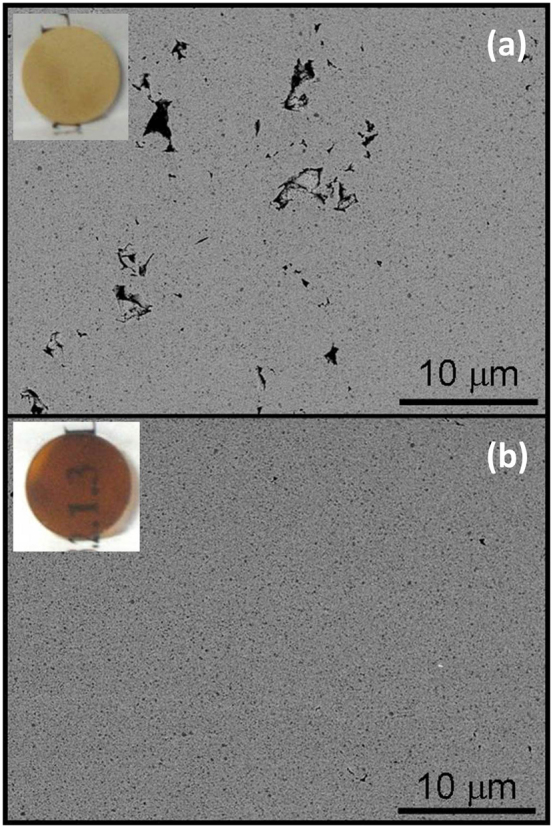
Cross sections were prepared using a cross-section polisher with an Ar ion beam. Insets show photographs of pelletized samples.
Figure 4 shows the relationship between the molding pressure and the ionic conductivity of the 75Li2S·25P2S5 glass. It shows that the ionic conductivity increases with increasing molding pressure; it increases dramatically to 10−4 S cm−1 at a molding pressure of 70 MPa and it then increases gradually. The ionic conductivity is 3.1 × 10−4 S cm−1 at a molding pressure of 360 MPa, which is very close to the bulk conductivity (3.4 × 10−4 S cm−1) of the 75Li2S·25P2S5 glass used in this study. (The bulk conductivity was measured for a transparent pellet prepared by hot pressing.) This change in the conductivity agrees well with the above finding that grain boundaries in 75Li2S·25P2S5 glass particles decreased remarkably on cold pressing. In contrast, Li7La3Zr2O12 has a relatively low conductivity that was difficult to measure due to the high grain boundary resistance after pressing at room temperature.
Figure 4. Relationship between molding pressure and ionic conductivity of 75Li2S·25P2S5 glass.
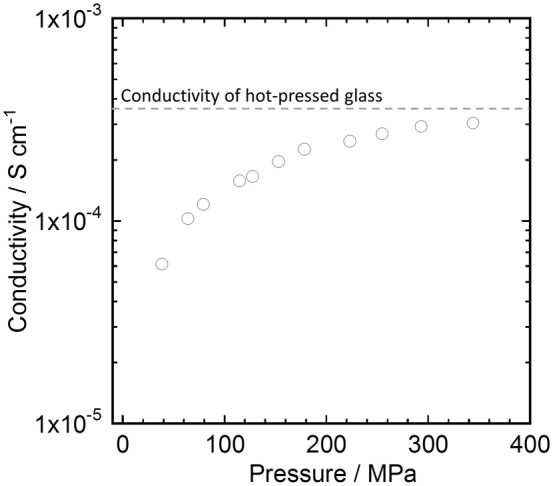
Dashed line indicates the conductivity of 75Li2S·25P2S5 bulk glass.
The elastic modulus of solid electrolytes is an important parameter when designing all-solid-state batteries. In lithium secondary batteries, the volume of electrode active materials changes during charging and discharging. This volume change results in fragmentation of the electrode particles and the formation of cracks, which cause capacity fading of batteries. In addition, since solid electrolytes cannot fill cracks, it is vital to suppress fragmentation in all-solid-state batteries. Stress generated by the volume change is concentrated on solid/solid interfaces, such as electrode/electrolyte and electrolyte/electrolyte interfaces. To prevent stress concentration at the interfaces, solid electrolytes should deform elastically. In this study, we first measured the Young's moduli of sulfide solid electrolytes with a high lithium-ion concentration.
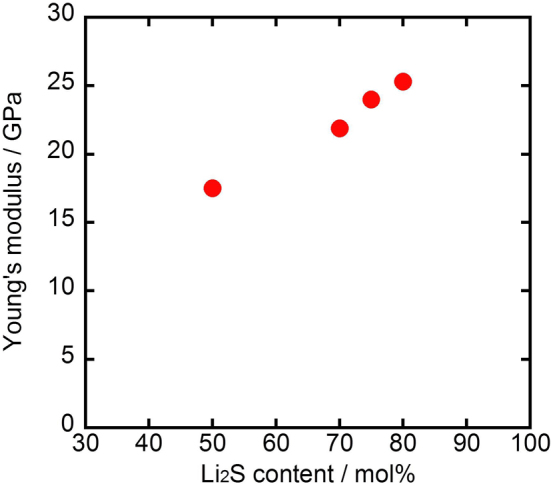
Figure 5 shows Young's moduli of Li2S–P2S5 glassy solid electrolytes for four different Li2S contents. High-density pellets were prepared by hot pressing at their respective glass transition temperatures. The Young's modulus of xLi2S·(100–x)P2S5 (mol.%) glasses (x = 50 − 80) increased with increasing Li2S content; the Young's moduli are in the range 18–25 GPa, which are much lower than those of oxide solid electrolytes. For example, the Young's modulus of 50Li2S·50P2S5 sulfide glass (18 Pa) is about one third that of 50Li2O·50P2O5 oxide glass (50 GPa)10. Alkali metal ions with a larger ionic radius have a lower Young's modulus; sodium thiophosphate (75Na2S·25P2S5) glass has a Young's modulus of 18 GPa, which is smaller than that of lithium thiophosphate (75Li2S· 25P2S5) glass (24 GPa).
Figure 5. Young's modulus of Li2S–P2S5 glassy solid electrolytes for four different Li2S contents.
Discussion
As mentioned above, sulfide solid electrolytes can be densified by pressing at room temperature. This densification indicates that atoms and/or ions diffuse in the electrolytes. The solid electrolytes are composed of lithium (Li+) and thiophosphate ions (PS43–), which are thought to diffuse at the particle boundaries on pressing at room temperature. As a result, the boundaries and voids at the adjacent particles decreased and we term this densification “room-temperature pressure sintering”.
Materials with low bond energies between cations and anions are favorable for room-temperature pressure sintering. The bond energy between Li and S in sulfide solid electrolytes (Li2S–P2S5) is lower than the bond energy between Li and O in oxide solid electrolytes (Li2O–P2O5).
Li–S bonds are considerably covalent in character. This intermediate bond character is important since Li+ and PS43– ions in sulfide solid electrolytes are considered to readily rotate and diffuse on the application of stress. The covalent character of Li–S bonds is partially retained during rotation and diffusion. It is thus important for these bonds to have a low bond energy and to be relatively covalent in character since there will be less electrostatic repulsion than in more ionic compounds. Consequently, sulfide electrolytes will exhibit small-scale plasticity, making them favorable materials for room-temperature pressure sintering.
A glassy structure is also important for room-temperature pressure sintering since it has a large free volume, which facilitates ion diffusion and rotation. Materials with low melting and glass transition temperatures generally have low bond energies. Therefore, reducing the glass transition temperature can be used as a guideline for developing highly processable solid electrolytes. Sulfide solid electrolytes are thus suitable for room-temperature pressure sintering. The local environment of sulfide ions is essentially unchanged by pressing. The X-ray diffraction pattern shows that the compressed pellets are basically amorphous. This indicates that the sulfide glasses were sintered at much lower temperature relative to the glass transition temperature.
Understanding the Young's modulus of solid electrolytes is essential for developing batteries. In a lithium-ion battery, the volumes of the electrode active material particles change during charging and discharging. Contact between the electrodes and solid electrolytes should be maintained during this change in volume since otherwise the battery capacity will drop. Thus, it will be favorable that solid electrolytes deform elastically in response to the volume change. Most all-solid-state batteries that employ sulfide solid electrolytes exhibit good cycle performance11,12.
It is also important to suppress fracturing and cracking of solid electrolytes. Ionic and covalent bonds are generally considered to be strong. Thus, glasses, ceramics, and ionic crystals tend to be hard and brittle and fracture easily on applying mechanical stress. In contrast, materials with low bond energies such as sulfide solid electrolytes are much softer than those with high bond energies (e.g., typical ionic crystals and solid oxide electrolytes). The Young's modulus of a glass can be estimated from the bond dissociation energy per unit volume and the ion packing density13. Since sulfides have a lower bond dissociation energy per unit volume and ion packing density than oxides; sulfide glasses and ceramics have lower Young's moduli than oxide glasses and ceramics. Therefore, sulfide solid electrolytes are considered to be suitable for all-solid-state batteries.
We have designed a favorable composite electrode for all-solid-state batteries by applying the mechanical properties of sulfide solid electrolytes. To achieve good battery performance, a composite electrode requires close contact between the electrode and the electrolyte and a lithium-ion conduction path between the positive and negative electrodes. Figure 6a shows a schematic diagram of a composite electrode consisting of electrode particles coated with a sulfide solid electrolyte. In this figure, the gray regions indicate electrode active material particles and the yellow regions represent the solid electrolyte coating. A suitable composite electrode having the two above-mentioned properties can be produced by pressing solid-electrolyte-coated electrode particles at room temperature. Figure 6b shows a cross-sectional SEM image of a compressed composite electrode consisting of LiCoO2 particles coated with a solid electrolyte coating of 80Li2S·20P2S5, which was deposited on the LiCoO2 particles by pulsed laser deposition (PLD)14. The composite electrode layer is very dense and very few voids are visible in the SEM image. The Li2S–P2S5 solid electrolyte is in close contact with the LiCoO2 positive electrode particles. As shown in Fig. 6a, a lithium-ion conduction path is formed among the electrode particles. Room-temperature pressure sintering of the solid electrolytes results in the formation of effective interfaces between the electrode and the solid electrolyte coatings. Furthermore, the solid electrolyte coating has a Young's modulus that is intermediate between those of typical oxide ceramics and organic polymers. The coating is thus expected to function as a buffer layer during the volume change of the electrode that occurs during charging and discharging. It will also help preserve the structure of the composite electrode because it has a higher mechanical strength than polymer electrolytes. Figure 6c shows charge–discharge curves for a current density of 11 mA g−1 of an all-solid-state battery consisting of a LiCoO2 positive electrode, a graphite negative electrode, and a Li2S–P2S5 solid electrolyte. The battery has a discharge capacity of 133 mAh g−1, which was calculated from the mass of the positive composite electrode (including the electrolyte). The composite electrodes of both SE-coated LiCoO2 and SE-coated graphite showed relatively good cycle performance (Supplementary Fig. S3).
Figure 6.
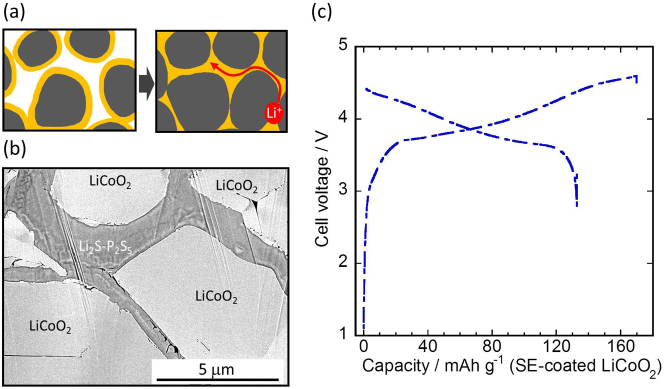
(a) Schematic diagram of composite electrode consisting of electrode particles coated with a solid sulfide electrolyte. Gray regions indicate electrode active material particles and yellow regions represent solid electrolyte coating. (b) Cross-sectional SEM image of compressed composite electrode consisting of LiCoO2 particles coated with 80Li2S·20P2S5 solid electrolyte. (c) Charge–discharge curves of all-solid-state battery consisting of a LiCoO2 positive electrode, a graphite negative electrode, and a Li2S–P2S5 solid electrolyte. The capacity was calculated from the mass of the positive composite electrode.
Developing a manufacturing process such as layer stacking of the components and realizing good contact between the electrode and solid electrolyte are important for producing all-solid-state batteries. Room-temperature pressure sintering is very promising for fabricating all-solid-state secondary batteries because it does not require high-temperature sintering. Since solid oxide electrolytes suffer from a high grain-boundary resistance, all-solid-state batteries with oxide solid electrolytes need to be sintered at high temperatures to reduce the grain boundary resistance. However, such high-temperature sintering sometimes induces side reactions at interfaces between the electrode and the solid electrolyte. Such side reactions can be prevented by using sulfide solid electrolytes because all-solid-state batteries can be fabricated by pressing at room temperature.
In summary, sulfide solid electrolytes have a high ionic conductivity, a good processability due to having a low bond energy, and a moderate Young's modulus. Sulfide solid electrolytes can be processed by room-temperature pressure sintering. Sulfide solid electrolytes are thus highly promising solid electrolytes for all-solid-state batteries. It has long been desired to increase the lithium-ion conductivity of solid electrolytes. The lithium-ion conductivities of some sulfide solid electrolytes have now reached those of organic liquid electrolytes currently used in practical lithium-ion batteries4,5. To develop all-solid-state batteries, it is important to determine the ideal mechanical properties for all-solid-state batteries and to develop new solid electrolytes with improved mechanical properties.
Methods
Preparation of Li2S–P2S5 and Na2S–P2S5 glass electrolytes
Li2S–P2S5 glasses were prepared by mechanochemical milling in a planetary ball mill (Fritsch, Pulverisette 7). The starting materials of Li2S (Idemitsu Kosan) and P2S5 (Aldrich) were hand ground and the mixture was then placed into a zirconia (ZrO2) vessel (internal volume of 45 ml) containing 500 4-mm-diameter ZrO2 balls. Li2S–P2S5 glass powders were formed by mechanochemical milling for 10 h at a fixed rotation speed of the base disc of 510 rpm. 75Na2S·25P2S5 (mol%) glass were prepared from Na2S (Nagao) by a similar procedure as that used to prepare the Li2S–P2S5 glasses. All these processes were performed in a dry Ar atmosphere.
The glass powder was compressed by conventional uniaxial cold pressing at room temperature or hot pressed at about 200°C to prepare 10-mm-diameter pellets. The bulk density of the sulfide solid electrolytes was determined from the density of the respective transparent glasses prepared by hot pressing because preparing Li2S–P2S5 bulk glass is quite difficult and has not been reported. The density of hot press glass was almost the same as that estimated from the density of the crystal. The relative density was calculated by dividing the density of the cold-pressed pellet by the density of the hot-pressed pellet.
Characterization of solid electrolytes
The microstructure of a cross section of the electrolyte pellets was observed by SEM (JEOL, JSM-6610A). Cross sections of the samples were prepared by fracturing or by using an ion milling system that employs an Ar ion beam (E-3500, Hitachi High-Technologies). Ion milling to obtain a smooth cross-section was conducted in a dry Ar atmosphere to prevent exposing the samples to air. Ultrasonic sound velocity measurements were performed to measure the room-temperature Young's modulus using the standard procedure for this method15. Dense hot-pressed pellets were used in these measurements. To prevent the sulfide solid electrolytes being exposed to air, ultrasonic measurements were conducted on samples in plastic bags. The supersonic measurement samples were 10 mm in diameter and several millimeters long.
The ionic conductivities of the compressed samples were measured using an impedance analyzer (Solartron, 147055BEC) in the frequency range of 10 Hz to 1 MHz with an applied voltage of 10 mV in a dry Ar gas atmosphere.
Evaluation of all-solid-state batteries
An all-solid-state test cell was fabricated using LiCoO2 as the positive electrode material, graphite as the negative electrode material, and 80Li2S·20P2S5 solid electrolytes. The LiCoO2 (D-10, Toda Kogyo) and graphite particles (Timrex SLP50, Timcal) were coated with the 80Li2S·20P2S5 solid electrolyte films by PLD14. The weight ratio of the LiCoO2 and 80Li2S·20P2S5 films in the positive composite electrode was ca. 90/10. Similarly, the weight ratio of the graphite and the 80Li2S·20P2S5 solid electrolyte in the negative composite electrode was ca. 90/10. Carbon was not added to the composite electrodes as a conducting additive. The electrodes (10 mg) and solid electrolyte (80 mg) powders were placed in a 10-mm-diameter polycarbonate tube and pressed together by applying a pressure of 360 MPa at room temperature to prepare an all-solid-state cell. The three-layered pellet was sandwiched between two stainless-steel rods as current collectors. All the cell preparation processes were performed in a dry Ar-filled glove box. Electrochemical tests were conducted at a constant current density of 0.13 mA cm−2 (ca. 11 mA g−1) in the voltage range from 2.8 to 4.6 V at room temperature under an Ar atmosphere using a charge–discharge measurement device.
Author Contributions
A.S. performed the experiments and wrote the paper. A.H. and M.T. designed the study and analyzed the data.
Supplementary Material
Supplemental Figures 1-3
Acknowledgments
The authors would like to thank Mr. Sumihisa Ishikawa of Toray Research Center, Inc. for assistance with obtaining the SEM images of the smooth cross-sections prepared by ion milling. The Young's moduli of the solid electrolytes were determined at Japan Fine Ceramics Center with technical support from Mr. Daisuke Igimi. The authors are also grateful to Prof. Kenji Higashi and Assoc. Prof. Yorinobu Takigawa of Osaka Prefecture University for fruitful discussions about the experimental measurements of the mechanical properties and analysis of the results. A.S. is grateful for a Grant-in-Aid and a fellowship from the Japan Society for the Promotion of Science (JSPS).
References
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). [DOI] [PubMed] [Google Scholar]
- Armand M. & Tarascon J.-M. Building better batteries. Nature 451, 652–657 (2008). [DOI] [PubMed] [Google Scholar]
- Mizuno F., Hayashi A., Tadanaga K. & Tatsumisago M. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv. Mater. 17, 918–921 (2005). [Google Scholar]
- Seino Y., Ota T., Jyunke T. & Yanagi K. Extend Abstracts of the 36th Symposium on Solid State Ionics in Japan, p. 116(2010) (in Japanese). [Google Scholar]
- Kamaya N. et al. A lithium superionic conductor. Nature Mater. 10, 682–686 (2011). [DOI] [PubMed] [Google Scholar]
- Hayashi A., Noi K., Sakuda A. & Tatsumisago M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nature Commun. 3, 856–860 (2012). [DOI] [PubMed] [Google Scholar]
- Murugan R., Thangadurai V., & Weppner W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007). [DOI] [PubMed] [Google Scholar]
- Hayashi A., Hama S., Morimoto H., Tatsumisago M. & Minami T. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 84, 477–479 (2001). [Google Scholar]
- Kitaura H., Hayashi A., Ohtomo T., Hama S. & Tatsumisago M. Fabrication of electrode-electrolyte interfaces in all-solid-state rechargeable lithium batteries by using a supercooled liquid state of the glassy electrolytes. J. Mater. Chem. 21, 118–124 (2011). [Google Scholar]
- Sokolov I. A., Il'in A. A., Tarlakov Y. P., Valova N. A. & Pronkin A. A. Structure and Physicochemical Properties of Glasses in the Li2S–LiPO3 System. Glass Phys. Chem. 29(3), 282–290 (2003). [Google Scholar]
- Minami T., Hayashi A. & Tatsumisago M. Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ionics 177, 2715–2720 (2006). [Google Scholar]
- Nagao M., Hayashi A. & Tatsumisago M. Sulfur–carbon composite electrode for all-solid-state Li/S battery with Li2S–P2S5 solid electrolyte. Electrochimica Acta 56, 6055–6059 (2011). [Google Scholar]
- Makishima A. & Mackenzie J. D. Direct calculation of Young's modulus of glass. J. Non-Crystal. Solids 12, 35–45 (1973). [Google Scholar]
- Sakuda A., Hayashi A., Ohtomo T., Hama S. & Tatsumisago M. LiCoO2 electrode particles coated with Li2S–P2S5 solid electrolyte for all-solid-state batteries. Electrochem. Solid-State Lett. 13(6), A73–A75 (2010). [Google Scholar]
- McSram H. J. Pulse Superposition Method for Measuring Ultrasonic Wave Velocities in Solids. J. Acoust. Soc. Am. 33, 12–16 (1961). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures 1-3