Abstract
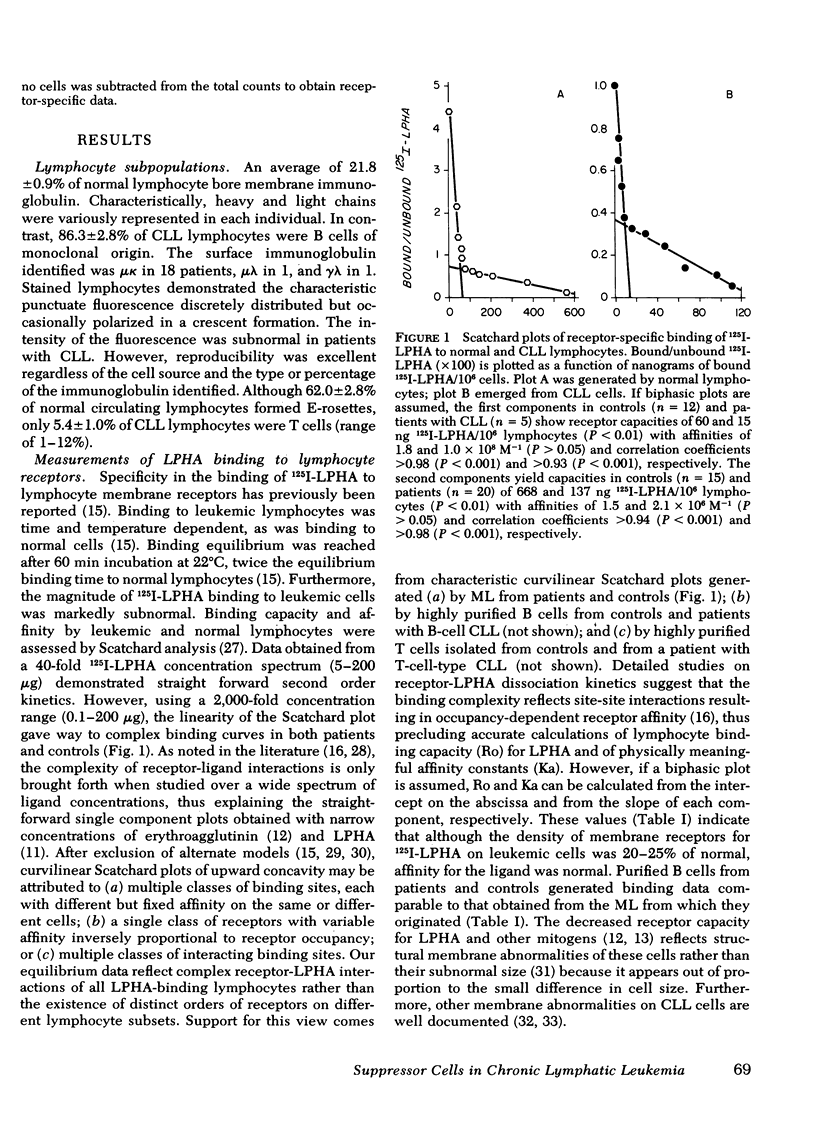
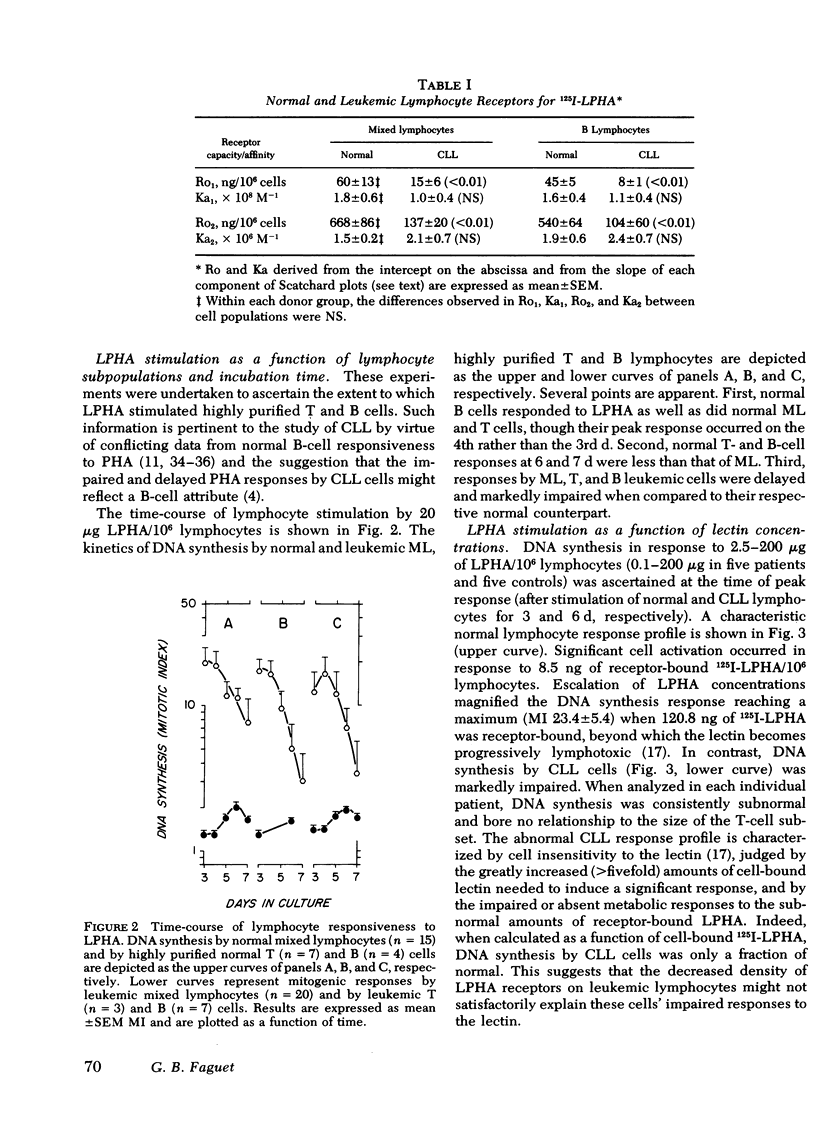
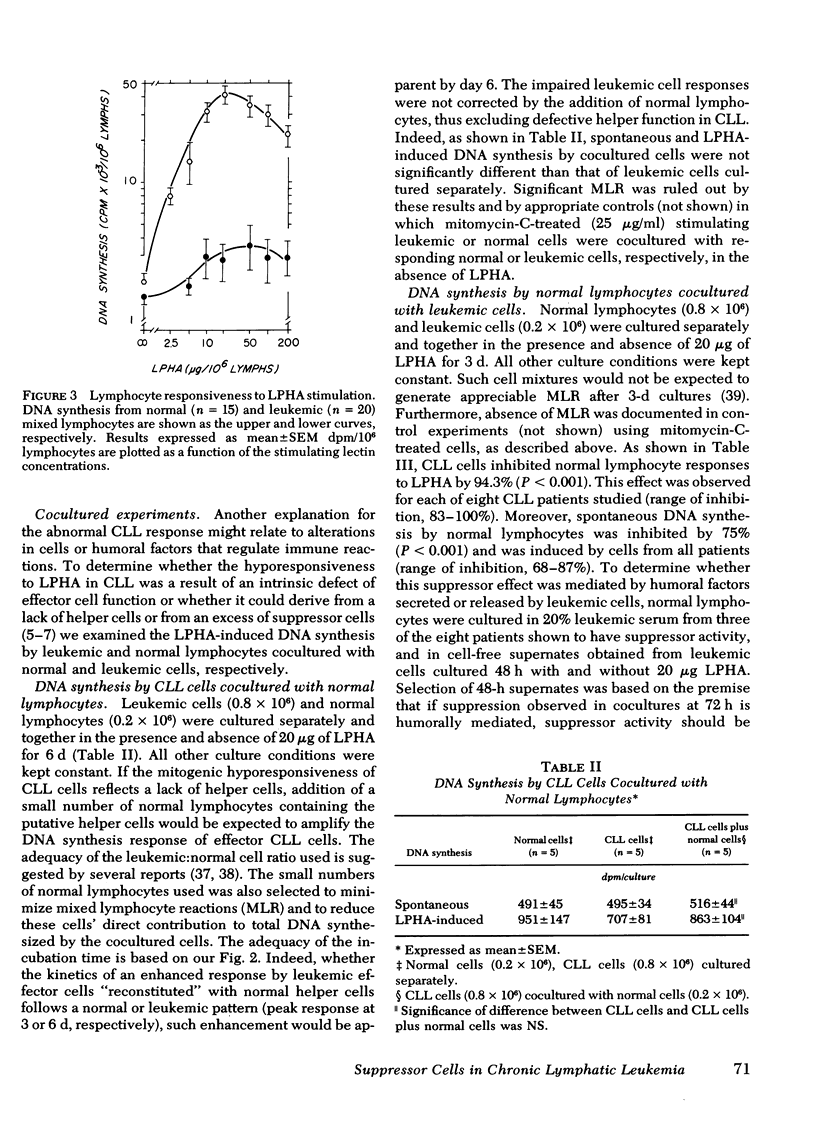
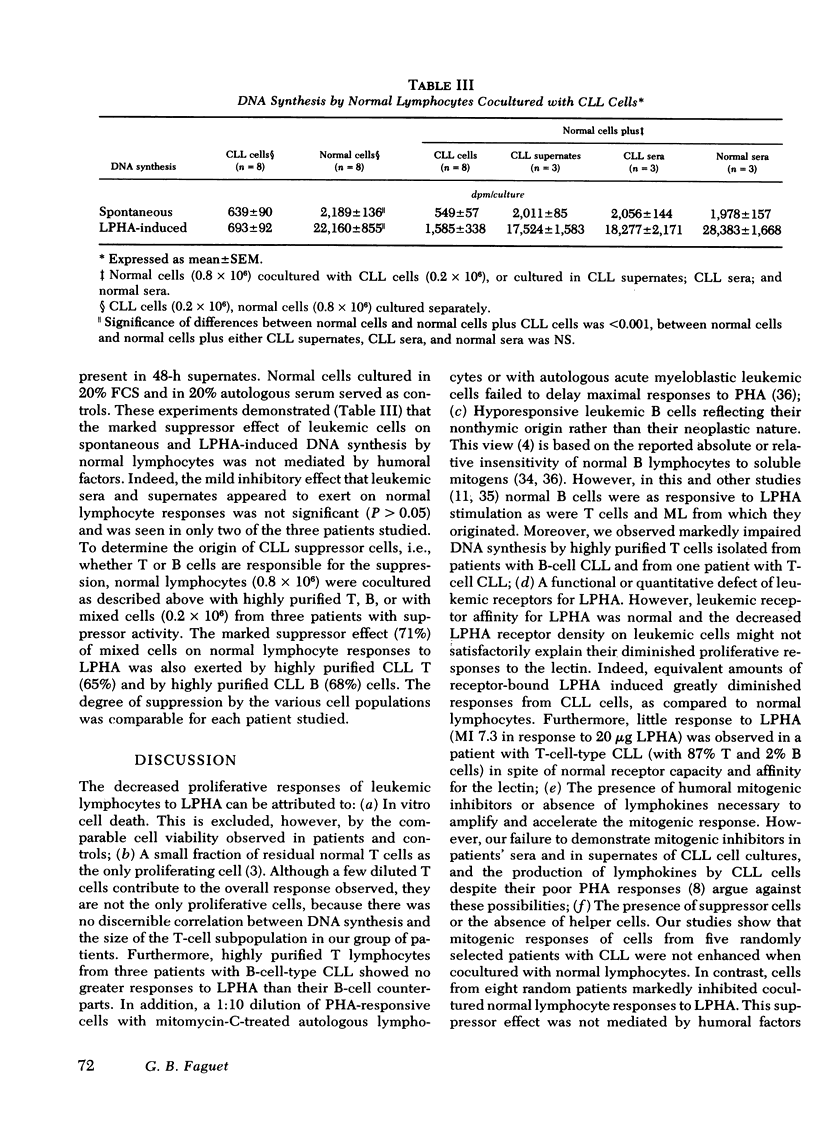
Binding of 125I-leukoagglutinin (LPHA) to lymphocyte membrane receptors at equilibrium generated similar curvilinear Scatchard plots in 20 patients with bursa-derived (B)-cell-type chronic lymphatic leukemia (CLL) and 15 controls. If biphasic plots are assumed, the two linear components show markedly diminished receptor capacity (15 and 137 ng/106 lymphocytes) in CLL as compared to controls (60 and 668 ng). In contrast, affinity was similar in patients (1.0 × 108 M−1 and 2.1 × 106 M−1) and controls (1.8 × 108 M−1 and 1.5 × 106 M−1). Highly purified B cells from patients and controls generated binding data comparable to that obtained from the mixed lymphocyte (ML) suspensions from which they originated. Maximal DNA synthesis of highly purified, normal, thymus-derived (T) and B cells in response to LPHA stimulation was comparable to that of ML (mitotic index [MI] 19.9, 20.1, and 23.4, respectively), though B-cell responses were slightly delayed. In CLL the markedly decreased and delayed DNA synthesis by ML (MI 2.3), and by highly purified T (MI 1.6) and B (MI 1.9) cells seemed out of proportion to their decreased receptor capacity for LPHA. The impaired mitogenic responses of leukemic cells from five patients were not enhanced when cocultured with normal lymphocytes. In contrast, cells from eight patients inhibited cocultured normal lymphocyte responses to LPHA by 94.3%. Sera from these patients and supernates from their cultured cells did not mediate this suppressor effect. These observations indicate that the decreased DNA synthesis observed in CLL is not an attribute of B cells and does not represent the expected response of a few residual normal T lymphocytes, but rather reflects impaired responses by all CLL cells. The defect does not relate to the density or function of membrane receptors for LPHA, to the presence of inhibitors in these patients' sera, or to depletion of helper T cells. Our data strongly suggest that one mechanism for the immunoincompetence observed in CLL reflects excessive suppressor-cell activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J. Immunoglobulins on the surface of neoplastic lymphocytes. N Engl J Med. 1972 Aug 10;287(6):272–276. doi: 10.1056/NEJM197208102870603. [DOI] [PubMed] [Google Scholar]
- Arpels C., Southam C. M. Cytotoxicity of sera from healthy persons and cancer patients. Int J Cancer. 1969 Jul 15;4(4):548–559. doi: 10.1002/ijc.2910040419. [DOI] [PubMed] [Google Scholar]
- BRODY J. I., BEIZER L. H. ALTERATION OF BLOOD GROUP ANTIGENS IN LEUKEMIC LYMPHOCYTES. J Clin Invest. 1965 Sep;44:1582–1589. doi: 10.1172/JCI105264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt D. H., MacDermott R. P., Jorolan E. P. Interaction of plant lectins with purified human lymphocyte populations: binding characteristics and kinetics of proliferation. J Immunol. 1975 May;114(5):1532–1536. [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Eggers A. E., Wunderlich J. R. Suppressor cells in tumor-bearing mice capable of nonspecific blocking of in vitro immunization against transplant antigens. J Immunol. 1975 May;114(5):1554–1556. [PubMed] [Google Scholar]
- Faguet G. B. Cellular immunity in sarcoidosis: evidence for an intrinsic defect of effector cell function. Am Rev Respir Dis. 1978 Jul;118(1):89–96. doi: 10.1164/arrd.1978.118.1.89. [DOI] [PubMed] [Google Scholar]
- Faguet G. B. Lymphocyte purification: an improved method. Qualitative and quantitative evaluation. Biomedicine. 1974 Apr 10;21(4):153–157. [PubMed] [Google Scholar]
- Faguet G. B. Mechanisms of lymphocyte activation. Binding kinetics of phytohemagglutinin to human lymphocytes. J Biol Chem. 1977 Mar 25;252(6):2095–2100. [PubMed] [Google Scholar]
- Faguet G. B. Mechanisms of lymphocyte activation: effect of ligand concentration and induction time. Adv Exp Med Biol. 1976;73(PT-A):329–337. doi: 10.1007/978-1-4684-3297-8_27. [DOI] [PubMed] [Google Scholar]
- Faguet G. B. Quantitation of immunocompetence in Hodgkin's disease. J Clin Invest. 1975 Oct;56(4):951–957. doi: 10.1172/JCI108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Rosen F. S., Merler E. Unresponsiveness of human B lymphocytes to phytohaemagglutinin. Nature. 1974 Mar 29;248(447):426–428. doi: 10.1038/248426a0. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunity to murine sarcoma virus-inducted tumors. II. Suppression of T cell-mediated immunity by cells from progressor animals. J Immunol. 1974 May;112(5):1826–1838. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S., Janossy G. Lymphocyte activation. 3. Binding sites for phytomitogens on lymphocyte subpopulations. Clin Exp Immunol. 1972 Mar;10(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. I., Teather C., Andrews H. Organizational difference of cell surface "hematoside" in normal and virally transformed cells. Biochem Biophys Res Commun. 1968 Nov 25;33(4):563–568. doi: 10.1016/0006-291x(68)90332-x. [DOI] [PubMed] [Google Scholar]
- Havemann K., Rubin A. D. The delayed response of c0ronic lymphocytic leukemia lymphocytes to phytohemagglutinin in vitro. Proc Soc Exp Biol Med. 1968 Mar;127(3):668–671. doi: 10.3181/00379727-127-32769. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakura S. MLC stimulatory capacity and production of blastogenic factor in patients with chronic lymphatic leukemia and Hodgkin's disease. Blood. 1975 Jun;45(6):823–832. [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Kontiainen S., Feldmann M. Suppressor cell induction in vitro. I. Kinetics of induction of antigen-specific suppressor cells. Eur J Immunol. 1976 Apr;6(4):296–301. doi: 10.1002/eji.1830060412. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Decreased phytohemagglutinin receptor sites in chronic lymphocytic leukemia. Biochim Biophys Acta. 1969 Dec 30;192(3):542–545. doi: 10.1016/0304-4165(69)90409-7. [DOI] [PubMed] [Google Scholar]
- Krakauer R. S., Strober W., Waldmann T. A. Hypogammaglobulinemia in experimental myeloma: the role of suppressor factors from mononuclear phagocytes. J Immunol. 1977 Apr;118(4):1385–1390. [PubMed] [Google Scholar]
- Krug U., Hollenberg M. D., Cuatrecasas P. Changes in the binding of concanavalin A and wheat germ agglutinin to human lymphocytes during in vitro transformation. Biochem Biophys Res Commun. 1973 May 1;52(1):305–312. doi: 10.1016/0006-291x(73)90988-1. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Biniamnov M., Ramot B., Katchalski E. Binding of concanavalin A to rat, normal human, and chronic lymphatic leukemia lymphocytes. Blood. 1972 Sep;40(3):311–316. [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Rai K. R., Sawitsky A., Cronkite E. P., Chanana A. D., Levy R. N., Pasternack B. S. Clinical staging of chronic lymphocytic leukemia. Blood. 1975 Aug;46(2):219–234. [PubMed] [Google Scholar]
- Rodbard D. Mathematics of hormone-receptor interaction. I. Basic principles. Adv Exp Med Biol. 1973;36(0):289–326. doi: 10.1007/978-1-4684-3237-4_14. [DOI] [PubMed] [Google Scholar]
- Rubin A. D., Havemann K., Dameshek W. Studies in chronic lymphocytic leukemia: further studies of the proliferative abnormality of the blood lymphocyte. Blood. 1969 Feb;33(2):313–328. [PubMed] [Google Scholar]
- Schrek R. Ultrastructure of blood lymphocytes from chronic lymphocytic and lymphosarcoma cell leukemia. J Natl Cancer Inst. 1972 Jan;48(1):51–64. [PubMed] [Google Scholar]
- Schultz E. F., Davis S., Rubin A. D. Further characterization of the circulating cell in chronic lymphocytic leukemia. Blood. 1976 Aug;48(2):223–234. [PubMed] [Google Scholar]
- Twomey J. J., Sharkey O., Jr, Brown J. A., Laughter A. H., Jordan P. H., Jr Cellular requirements for the mitotic response in allogeneic mixed leukocyte cultures. J Immunol. 1970 Apr;104(4):845–853. [PubMed] [Google Scholar]
- Waldmann T. A., Blaese R. M., Broder S., Krakauer R. S. Disorders of suppressor immunoregulatory cells in the pathogenesis of immunodeficiency and autoimmunity. Ann Intern Med. 1978 Feb;88(2):226–238. doi: 10.7326/0003-4819-88-2-226. [DOI] [PubMed] [Google Scholar]
- Weber T. H. Isolation and characterization of a lymphocyte-stimulating leucoagglutinin from red kidney beans. (Phaseolus vulgaris). Scand J Clin Lab Invest Suppl. 1969;111:1–80. [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]


