Abstract
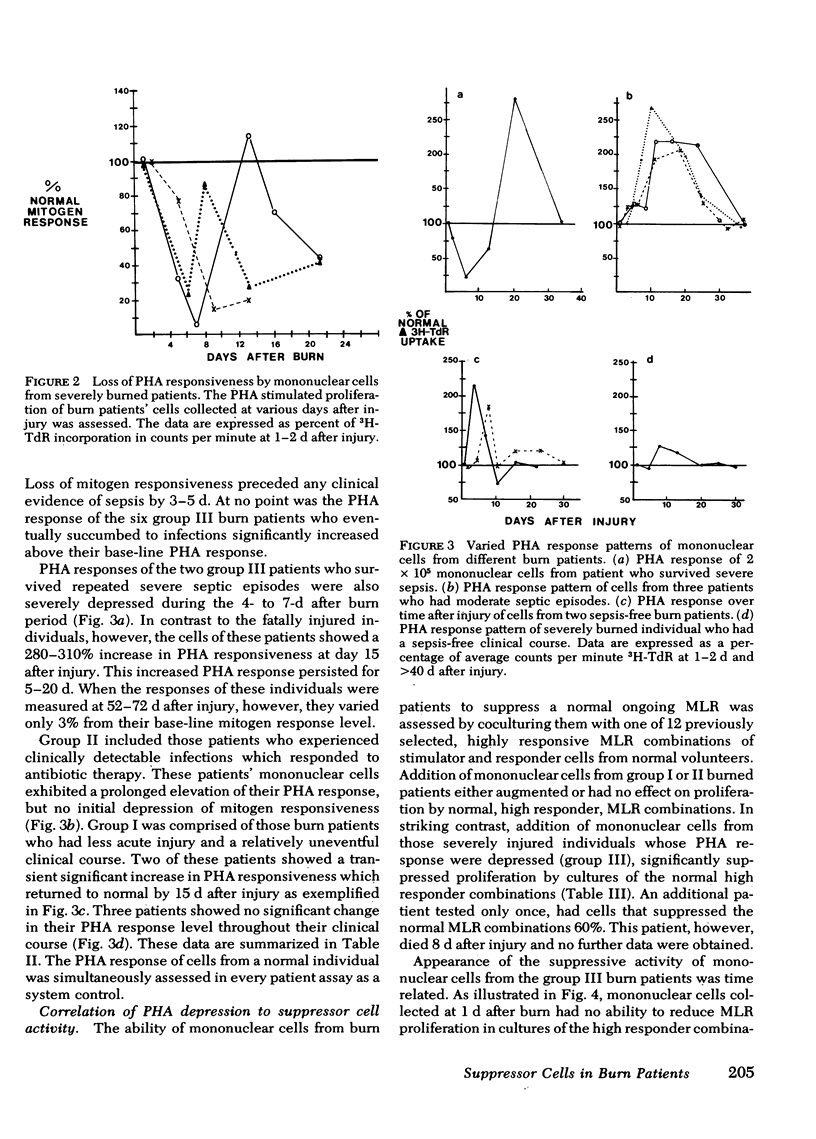
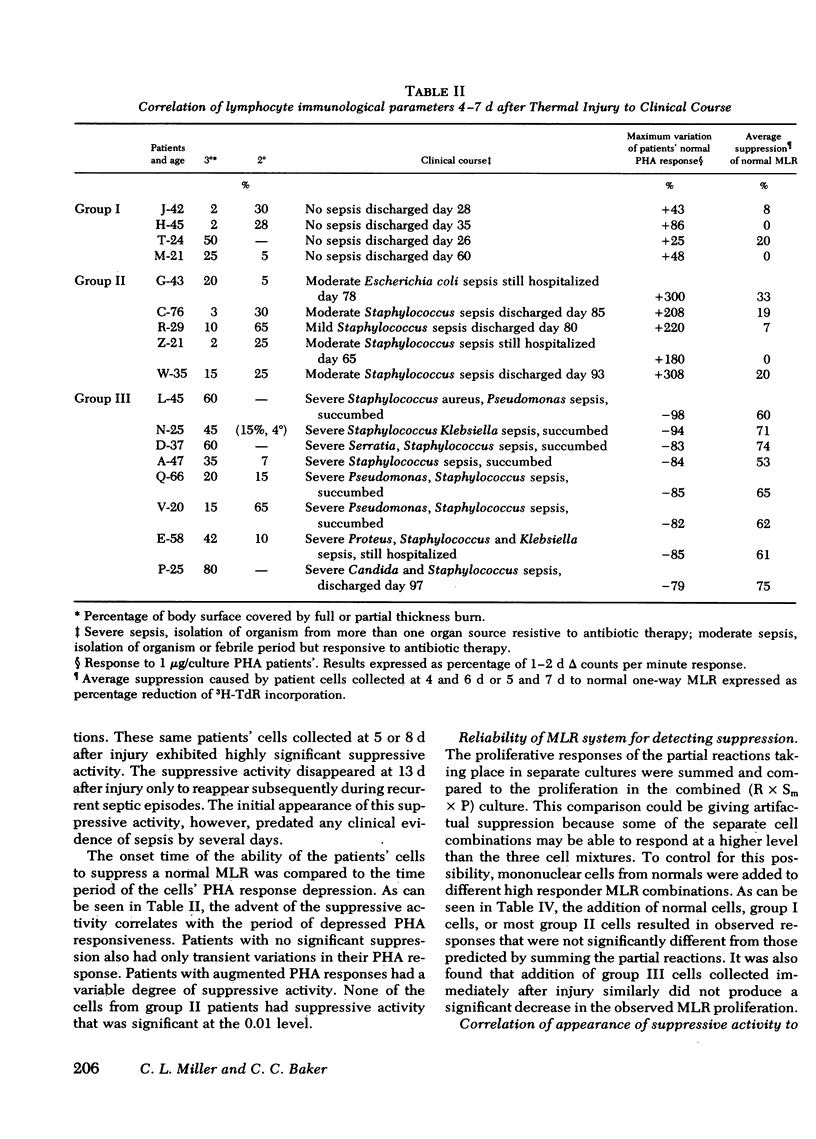
The high incidence of fatal septicemia associated with severe thermal injury is believed to result from a loss of immunocompetence. To detect burn-mediated immune defects, lymphocyte function in peripheral blood leukocytes from 18 individuals sustaining 20-80% full thickness thermal burns was investigated. We examined the kinetics of the mitogen responses, the development of suppressive activity, and the correlation of mononuclear cell functional abnormalities with the incidence of sepsis. Patients were divided into three groups corresponding to their clinical course. The phytohemagglutinin responses of Ficoll-Hypaque purified leukocytes from eight of these patients (group III) were normal at day 1-2 after injury, but were significantly depressed (mean 16% of normal) at days 5-10 after injury. All of these group III patients experienced multiple, severe, septic episodes, and septic mortality was 75%. The other 10 burned individuals showed either augmented (group II) or unaltered (group I) mitogen responsiveness.
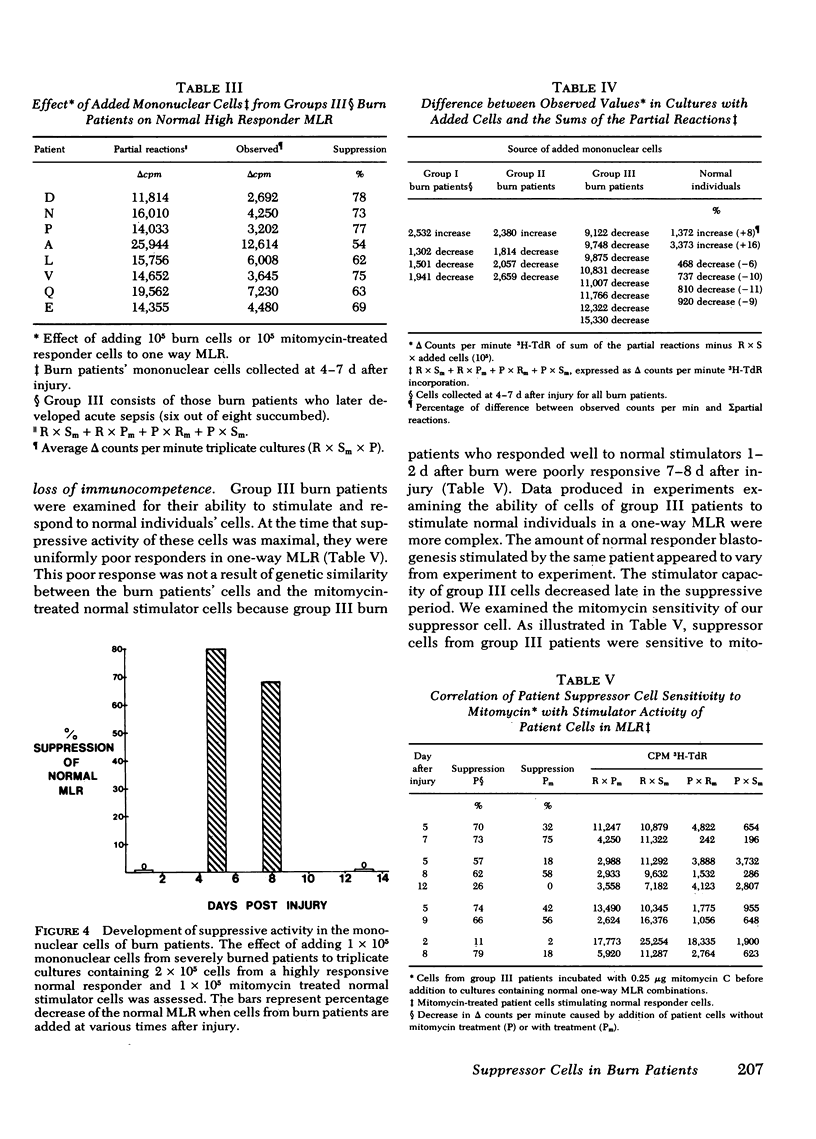
Concomitant with evaluation of their mitogen responses, the cells of burn patients were assessed for development of suppressive activity by addition to on-going normal mixed leukocyte reactions (MLR). Only the addition of mononuclear cells with depressed phytohemagglutinin responsiveness (group III) significantly decreased MLR proliferation (mean 80% reduction) by the previously highly responsive, normal MLR combinations. Addition of cells from group III burn patients collected immediately after injury had no suppressive effect. Addition of cells from patients in group I or II or of normal individual's cells had no suppressive effect. These experimental results strongly suggest that a suppressive mononuclear cell is at least partially responsible for the decreased immunocompetence of burn patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Furukawa C. T., Klebanoff S. J. Depressed mononuclear leukocyte chemotaxis in thermally injured patients. J Immunol. 1977 Jul;119(1):199–205. [PubMed] [Google Scholar]
- Baxter C. R. The current status of burn research. J Trauma. 1974 Jan;14(1):1–8. [PubMed] [Google Scholar]
- Berlinger N. T., Lopez C., Good R. A. Facilitation or attenuation of mixed leukocyte culture responsiveness by adherent cells. Nature. 1976 Mar 11;260(5547):145–146. doi: 10.1038/260145a0. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Constantian M. B. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978 Aug;188(2):209–215. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J. C., Cobb E. K., Lynch J. B., Lewis S. R., Larson D. L., Ritzmann S. E. Altered nucleic acid synthesis in lymphocytes from patients with thermal burns. Surg Gynecol Obstet. 1970 May;130(5):783–788. [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Cobb E. K., Lewis S. R., Larson D. L., Ritzmann S. E. Evaluation of lymphocyte reactivity studies in patients with thermal burns. J Trauma. 1971 Jul;11(7):595–601. doi: 10.1097/00005373-197107000-00011. [DOI] [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Ritzmann S. E. Lymphoid response of the burn patient. South Med J. 1975 Jul;68(7):865–870. [PubMed] [Google Scholar]
- DuPont B., Hansen J. A. Human mixed-lymphocyte culture reaction: genetics, specificity, and biological implications. Adv Immunol. 1976;23:107–202. doi: 10.1016/s0065-2776(08)60320-x. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Howard R. J., Simmons R. L. Acquired immunologic deficiencies after trauma and surgical procedures. Surg Gynecol Obstet. 1974 Nov;139(5):771–782. [PubMed] [Google Scholar]
- Kohn J. Abnormal immune response in burns. Postgrad Med J. 1972 Jun;48(560):335–337. [PMC free article] [PubMed] [Google Scholar]
- Krizek T. J., Cossman D. V. Experimental burn wound sepsis: variations in response to topical agents. J Trauma. 1972 Jul;12(7):553–562. [PubMed] [Google Scholar]
- Leguit P., Jr, Meinesz A., Zeijlemaker W. P., Schellekens P. T., Eijsvoogel V. P. Immunological studies in burn patients. I. Lymphocyte transformation in vitro. Int Arch Allergy Appl Immunol. 1973;44(1):101–121. doi: 10.1159/000230921. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler D., Batchelor J. R. Phytohaemagglutinin transformation of lymphocytes in burned patients. Transplantation. 1971 Nov;12(5):409–411. doi: 10.1097/00007890-197111000-00015. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E. T. Effect of thermal trauma on numbers and function of T and B cells from mouse spleen. Int Arch Allergy Appl Immunol. 1977;54(3):238–246. doi: 10.1159/000231832. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E. T., LaJohn L. A. The effect of thermal trauma in mice on cytotoxicity of lymphocytes. Proc Soc Exp Biol Med. 1977 Jan;154(1):72–77. doi: 10.3181/00379727-154-39607. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T. A suppressor T cell in the human mixed lymphocyte reaction. J Exp Med. 1977 Aug 1;146(2):368–380. doi: 10.1084/jem.146.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Trunkey D. D. Thermal injury: defects in immune response induction. J Surg Res. 1977 Jun;22(6):621–625. doi: 10.1016/0022-4804(77)90100-7. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Eurenius K., Katz R. M., Canales L., Foley F. D., Mortensen R. F. Cell-mediated immunity after thermal injury. Ann Surg. 1973 Feb;177(2):139–143. doi: 10.1097/00000658-197302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M. Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet. 1976 Jun 19;1(7973):1329–1330. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- Neilan B. A., Taddeini L., Strate R. G. T lymphocyte rosette formation after major burns. JAMA. 1977 Aug 8;238(6):493–496. [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J. Kinetics of humoral responsiveness in severe thermal injury. Ann Surg. 1976 Jul;184(1):51–59. doi: 10.1097/00000658-197607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Daniels J. C., Beathard G. A., Lewis S. R., Lynch J. B., Ritzmann S. E. Mixed lymphocyte culture reaction in patients with acute thermal burns. J Trauma. 1974 Jan;14(1):53–57. doi: 10.1097/00005373-197401000-00007. [DOI] [PubMed] [Google Scholar]
- Sampson D., Grotelueschen C., Kauffman H. M., Jr The human splenic suppressor cell. Transplantation. 1975 Nov;20(5):362–367. doi: 10.1097/00007890-197511000-00002. [DOI] [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Bankhurst A. D., Williams R. C., Jr Studies of cell subpopulations mediating mitogen hyporesponsiveness in patients with Hodgkin's disease. J Clin Invest. 1978 Jan;61(1):55–63. doi: 10.1172/JCI108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Smith C. W., Goldman A. S. Selective effects of thermal injury on mouse peritoneal macrophages. Infect Immun. 1972 Jun;5(6):938–941. doi: 10.1128/iai.5.6.938-941.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger L. A., Nowell P. C., Daniele R. P. Sequential proliferation induced in human peripheral blood lymphocytes by mitogen. II. Suppression by PHA-activated cells. J Immunol. 1977 May;118(5):1768–1773. [PubMed] [Google Scholar]


