Abstract
The novel two-component systems NsrRS and LcrRS are individually associated with resistance against the distinct lantibiotics nisin A and nukacin ISK-1 in Streptococcus mutans. NsrRS regulates the expression of NsrX, which is associated with nisin A binding, and LcrRS regulates the expression of the ABC transporter LctFEG.
TEXT
Streptococcus mutans, a commensal bacterium in the oral cavity, is a cariogenic pathogen in humans (1, 2, 3). S. mutans forms dental plaques with other bacterial species. Therefore, S. mutans has evolved mechanisms that facilitate competition or cooperation with other oral bacteria in dental plaques. Many bacteria produce antibacterial agents, known as bacteriocins, to ensure survival within this community (4, 5). Bacteriocins are primarily classified into classes I and II (6). Class I bacteriocins (peptides of <5 kDa), called lantibiotics, contain a ring bridged by lanthionine and 3-methyllanthionine residues (7), whereas class II bacteriocins comprise unmodified amino acids (8). S. mutans produces several types of bacteriocins known as mutacins (9–12). However, the mechanism underlying the resistance of S. mutans to the bacteriocins of other bacteria has not been elucidated. Recently, two-component system (TCS)-based resistance mechanisms against antibacterial agents, including bacteriocins, have been identified in several bacterial species (13–17). In this study, we evaluated the roles of the S. mutans TCSs in resistance to several types of bacteriocins.
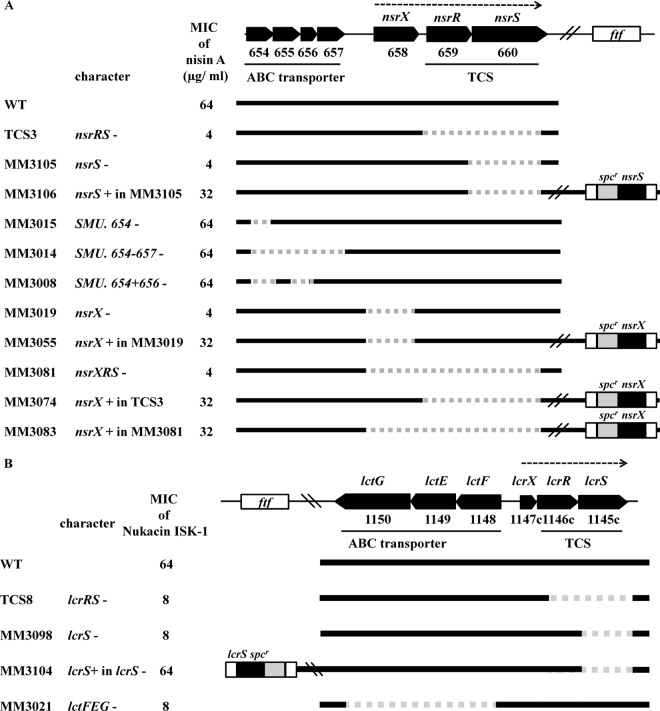
We first examined 14 S. mutans TCS mutants for susceptibility to class I and II bacteriocins by using previously described direct and MIC methods (18, 19) (Table 1 and Fig. 1). The strains, plasmids, and primers are listed in Tables S1 and S2 in the supplemental material. The methods for the construction of the mutants are described in the supplemental materials. Nisin A and nukacin ISK-1 were purified as previously described (20). One TCS encoded by the SMU.659-660 mutant (designated nsrRS [nisin A-resistant TCS] in this study) displayed increased susceptibility to nisin A compared with the wild type. Because we identified the function of this TCS, previously named SpaKR (21), we designated this TCS NsrRS. Another TCS encoded by the SMU.1146-1145 mutant (designated lcrRS [lacticin 481-resistant TCS]) displayed increased susceptibility to nukacin ISK-1 and lacticin 481. Each complemented strain was able to restore the respective mutation (Fig. 1). The susceptibilities of all TCS mutants against individual class II bacteriocins were not significantly different than those of wild-type cells.
Table 1.
Susceptibilities of TCS deletion mutants to various bacteriocins
| Strain | Susceptibilitya to: |
||||||
|---|---|---|---|---|---|---|---|
| Class I |
Class IIa, munditicin | Class IIb, mutacin IV | Class IIc, lactocyclicin Q | Class IId, lacticin Q | |||
| Nisin A | Nukacin ISK-I | Lacticin 481 | |||||
| UA159 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS45 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS1 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS2 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS3 | 8 (4) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS4 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS5 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS6 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS7 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS8 | 1 (64) | 8 (8) | 7 | 5 | 0 | 3 | 8 |
| TCS9 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS10 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS11 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS12 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS13 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
| TCS14 | 1 (64) | 1 (64) | 1 | 5 | 0 | 3 | 8 |
Susceptibility was based on direct analysis (the diameter of inhibition [in mm]), with the exception of the two class I drugs on which this study was focused, nisin and nukacin ISK-I, for which MICs were also determined (MIC values [in μg/ml] are shown in parentheses).
Fig 1.
Evaluation of nisin A and nukacin ISK-1 susceptibilities. The characteristics of the nsrRS (A) and lcrRS (B) loci in the mutant strains used in this study are indicated. The dotted arrows indicate the same operon. The MICs of nisin A and nukacin ISK-1 are also shown.
DNA microarray experiments were performed to characterize the transcriptional control mediated by NsrRS and LcrRS. The Agilent eArray platform was used to design a microarray; 14,028 probes (60-mers) were designed for the 2,012 protein-coding genes of S. mutans UA159 (up to seven probes per gene) (detailed methods are provided in the supplemental materials). A comparison of the transcription profiles of UA159 and nsrRS mutant cells revealed that the nisin A-mediated induction of seven of these genes (including SMU.654-657, which encodes an ABC transporter, and nsrXRS) was reduced in the nsrRS mutant (Table 2). A comparison of the transcription profiles of UA159 and lcrRS mutants demonstrated that the nukacin ISK-1-mediated induction of six of these genes (including lctEFG, which encodes an ABC transporter, and lcrXRS) was reduced in the lcrRS mutant (Table 2). We further confirmed the expression of these genes by using quantitative PCR (data not shown).
Table 2.
Genes up- or downregulated in S. mutans UA159 following nisin A or nukacin ISK-1 treatment
| Treatment, regulation change, and gene IDa | Gene name | Regulation by TCS | Fold differenceb | Gene function |
|---|---|---|---|---|
| Nisin A | ||||
| Upregulated genes | ||||
| SMU.655 | mutE | +c | 17.3 | ABC transporter, permease |
| SMU.1961 | levD | − | 17.1 | Fructose-specific enzyme IIA component |
| SMU.654 | mutF | + | 16.8 | ABC transporter, ATP binding |
| SMU.656 | mutE2 | + | 14.8 | ABC transporter, ATP binding |
| SMU.657 | mutG | + | 14.6 | ABC transporter, permease |
| SMU.658 | nsrX | + | 12.9 | Conserved hypothetical protein |
| SMU.1958 | levF | − | 10.8 | Fructose-specific enzyme IIC component |
| SMU.659 | nsrR | + | 10.2 | Response regulator |
| SMU.1957 | levG | − | 9.0 | Fructose-specific enzyme IID component |
| SMU.1960 | levE | − | 7.6 | Fructose-specific enzyme IIB component |
| SMU.1956 | − | 7.2 | Conserved hypothetical protein | |
| SMU.660 | nsrS | + | 6.3 | Histidine kinase |
| SMU.1195 | − | 4.0 | ABC transporter permease protein | |
| SMU.1193 | yhcF | − | 3.9 | Transcriptional regulator, GntR family |
| SMU.1194 | yurY | − | 3.9 | ABC transporter, ATP binding |
| SMU.862 | − | 3.5 | Conserved hypothetical protein | |
| Nukacin ISK-1 | ||||
| Upregulated genes | ||||
| SMU.1148 | lctF | +d | 74.4 | ABC transporter, ATP binding |
| SMU.1149 | lctE | + | 67.3 | ABC transporter, permease |
| SMU.1150 | lctG | + | 53.3 | ABC transporter, permease |
| SMU.1705 | − | 6.5 | Conserved hypothetical protein | |
| SMU.1706 | − | 5.7 | Conserved hypothetical protein | |
| SMU.1704 | − | 5.2 | Conserved hypothetical protein | |
| SMU.1147 | lcrX | + | 4.7 | Hypothetical protein |
| SMU.1146 | lcrR | + | 4.4 | Response regulator |
| SMU.1145 | lcrS | + | 4.2 | Histidine kinase |
| SMU.753 | − | 4.1 | Conserved hypothetical protein | |
| Downregulated genes | ||||
| SMU.1960 | levE | − | 0.5 | Fructose-specific enzyme IIB component |
| SMU.1961 | levD | − | 0.3 | Fructose-specific enzyme IIA component |
| SMU.1877 | manL | − | 0.3 | Mannose PTS component IIAB |
| SMU.1878 | manM | − | 0.3 | Mannose PTS component IIC |
| SMU.1879 | manN | − | 0.3 | Mannose PTS component IID |
Gene identification (ID) numbers are from the GEO database of NCBI (http://www.ncbi.nlm.nhi.gov/geo/).
The fold change between bacteriocin-treated cells versus nontreated cells.
Regulated by NsrRS.
Regulated by LcrRS.
To identify the genes directly involved in nisin A resistance, we constructed SMU.654-657 and nsrXRS deletion mutants. The nsrXRS deletion mutant displayed increased susceptibility to nisin A, while the susceptibilities of the three SMU.654-657-related mutants were not altered (Fig. 1A). We also constructed an nsrX single mutant through substitution with the terminatorless Emr gene. We confirmed that the mutation did not affect nsrRS expression downstream of nsrX (data not shown). Similar to the nsrXRS deletion mutant, the nsrX single mutant also displayed increased susceptibility to nisin A. The complementation of nsrX in the nsrX single mutant and nsrXRS mutant restored nsrX expression and susceptibility to nisin A (Fig. 1A). These results indicated that nsrX is associated with the NsrRS-regulated resistance to nisin A. NsrX comprises 280 amino acids and nine predicted transmembrane helices. NsrX does not show homology with any other known immunity proteins, such as NukH, NisI, or LtnI (22, 23, 24). Instead, NsrX shares homology with several acetyltransferases, including those of the TraX family, which is associated with F pilin acetylation in Escherichia coli (25). Thus, we performed a nisin A-binding assay to characterize the binding of nisin A to S. mutans cells, using a previously described method with some modifications (22, 26). The results of the nisin A-binding assay revealed that more nisin A bound to MM3055 cells (an nsrX deletion mutant chromosomally complemented with the cloned nsrX gene) than to MM3019 cells (an nsrX deletion mutant) (Table 3). This finding suggested that NsrX or an as-yet-unidentified factor modified through NsrX binds to nisin A and inhibits the binding of nisin A to lipid II. To identify the genes that are directly involved in nukacin ISK-1 resistance, we constructed an lctFEG deletion mutant, which exhibited a reduced MIC against nukacin ISK-1 (Fig. 1B). We determined that the LcrRS-LctFEG system is involved in lacticin 481 resistance in Lactococcus lactis CNRZ481 (Table 2). The structure of lacticin 481 is similar to nukacin ISK-1 (6), indicating that LcrRS responds to the lacticin 481 group of type AII lantibiotics.
Table 3.
Quantitative nisin A-binding assay results with the nsrX deletion mutant and nsrX-expressing cells
| Strain | Genotype | Fraction | Nisin A bindinga (μg/ml) | P valueb |
|---|---|---|---|---|
| MM3019 | nsrX mutant | Bound | 10.7 ± 0.11 | |
| MM3055 | nsrX+ in nsrX mutant | Bound | 17.8 ± 0.23 | 0.008 |
| MM3019 | nsrX mutant | Unbound | 21.1 ± 0.70 | |
| MM3055 | nsrX+ in nsrX mutant | Unbound | 16.5 ± 0.94 | 0.015 |
Mean ± standard deviation binding activity, as measured by liquid chromatography/mass spectrometry.
Statistical significance for the difference between the nsrX deletion mutant versus the nsrX-expressing strain, based on Student's t test.
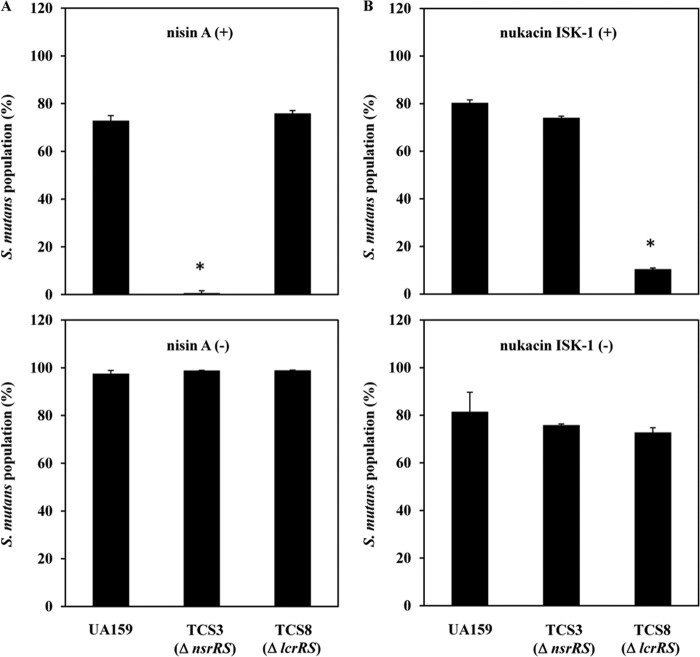
We performed a coculture assay using the method described in the supplemental material to determine whether TCS-mediated resistance was directly involved in colocalization with other bacteriocin-producing bacteria. S. mutans UA159 wild-type, nsrRS mutant, or lcrRS mutant cells were cocultured with nisin A-producing or nonproducing L. lactis strains. The nsrRS mutant exhibited dramatically decreased population ratios than wild-type or lcrRS mutant cells when cocultured with the nisin A-producing strain (L. lactis ATCC 11454) but not when cocultured with the non-nisin A-producing L. lactis strain (Fig. 2A). Similar results were obtained when S. mutans UA159 and mutant cells were cocultured with the nukacin ISK-1-producing or nonproducing strains (Fig. 2B).
Fig 2.
Coculture of S. mutans with L. lactis or Staphylococcus warneri. The methods for the coculture assay are described in the supplemental material. Populations of S. mutans were determined after L. lactis (A) or S. warneri (B) cells were cocultured with UA159, nsrRS mutant, or lcrRS mutant cells. *, P < 0.01, as determined using Dunnett's method, for the percentage of the S. mutans population.
In conclusion, we identified two novel TCSs involved in resistance to nisin A and nukacin ISK-1 in S. mutans and demonstrated that these two TCSs are important for coexistence with other class I bacteriocin-producing bacteria. These results highlight the roles of bacteriocins in the interactions between different species of oral bacteria and the importance of TCSs in these interactions.
Supplementary Material
ACKNOWLEDGMENT
This study was supported in part by Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan.
Footnotes
Published ahead of print 24 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00780-13.
REFERENCES
- 1. Hamada S, Slade HD. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuramitsu HK. 1993. Virulence factors of mutans streptococci: role of molecular genetics. Crit. Rev. Oral Biol. Med. 4:159–176 [DOI] [PubMed] [Google Scholar]
- 3. van Houte J. 1994. Role of micro-organisms in caries etiology. J. Dent. Res. 73:672–681 [DOI] [PubMed] [Google Scholar]
- 4. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 5. Nissen-Meyer J, Nes IF. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 167:67–77 [PubMed] [Google Scholar]
- 6. Bierbaum G, Sahl HG. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 10:2–18 [DOI] [PubMed] [Google Scholar]
- 7. Nagao J, Asaduzzaman SM, Aso Y, Okuda K, Nakayama J, Sonomoto K. 2006. Lantibiotics: insight and foresight for new paradigm. J. Biosci. Bioeng. 102:139–149 [DOI] [PubMed] [Google Scholar]
- 8. Nes IF, Holo H. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50–61 [DOI] [PubMed] [Google Scholar]
- 9. Qi F, Chen P, Caufield PW. 2000. Purification and biochemical characterization of mutacin I from the group I strain of Streptococcus mutans, CH43, and genetic analysis of mutacin I biosynthesis genes. Appl. Environ. Microbiol. 66:3221–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caufield PW, Shah G, Hollingshead SK, Parrot M, Lavoie MC. 1990. Evidence that mutacin II production is not mediated by a 5.6-kb plasmid in Streptococcus mutans. Plasmid 24:110–118 [DOI] [PubMed] [Google Scholar]
- 11. Qi F, Chen P, Caufield PW. 2001. The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl. Environ. Microbiol. 67:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robson CL, Wescombe PA, Klesse NA, Tagg JR. 2007. Isolation and partial characterization of the Streptococcus mutans type AII lantibiotic mutacin K8. Microbiology 153:1631–1641 [DOI] [PubMed] [Google Scholar]
- 13. Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. 2011. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 81:602–622 [DOI] [PubMed] [Google Scholar]
- 14. Tsuda H, Yamashita Y, Shibata Y, Nakano Y, Koga T. 2002. Genes involved in bacitracin resistance in Streptococcus mutans. Antimicrob. Agents Chemother. 46:3756–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouyang J, Tian XL, Versey J, Wishart A, Li YH. 2010. The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob. Agents Chemother. 54:3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majchrzykiewicz JA, Kuipers OP, Bijlsma JJ. 2010. Generic and specific adaptive responses of Streptococcus pneumoniae to challenge with three distinct antimicrobial peptides, bacitracin, LL-37, and nisin. Antimicrob. Agents Chemother. 54:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jordan S, Junker A, Helmann JD, Mascher T. 2006. Regulation of LiaRS-dependent gene expression in Bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J. Bacteriol. 188:5153–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barefoot SF, Klaenhammer TR. 1983. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 45:1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komatsuzawa H, Fujiwara T, Nishi H, Yamada S, Ohara M, McCallum N, Berger-Bachi B, Sugai M. 2004. The gate controlling cell wall synthesis in Staphylococcus aureus. Mol. Microbiol. 53:1221–1231 [DOI] [PubMed] [Google Scholar]
- 20. Sashihara T, Kimura H, Higuchi T, Adachi A, Matsusaki H, Sonomoto K, Ishizaki A. 2000. A novel lantibiotic, nukacin ISK-1, of Staphylococcus warneri ISK-1: cloning of the structural gene and identification of the structure. Biosci. Biotechnol. Biochem. 64:2420–2428 [DOI] [PubMed] [Google Scholar]
- 21. Song L, Sudhakar P, Wang W, Conrads G, Brock A, Sun J, Wagner-Döbler I, Zeng AP. 2012. A genome-wide study of two-component signal transduction systems in eight newly sequenced mutans streptococci strains. BMC Genomics 13:128. 10.1186/1471-2164-13-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okuda K, Aso Y, Nagao J, Shioya K, Kanemasa Y, Nakayama J, Sonomoto K. 2005. Characterization of functional domains of lantibiotic-binding immunity protein, NukH, from Staphylococcus warneri ISK-1. FEMS Microbiol. Lett. 250:19–25 [DOI] [PubMed] [Google Scholar]
- 23. Stein T, Heinzmann S, Solovieva I, Entian KD. 2003. Function of Lactococcus lactis nisin immunity genes nisI and nisFEG after coordinated expression in the surrogate host Bacillus subtilis. J. Biol. Chem. 278:89–94 [DOI] [PubMed] [Google Scholar]
- 24. McAuliffe O, Hill C, Ross RP. 2000. Identification and overexpression of ltnl, a novel gene which confers immunity to the two-component lantibiotic lacticin 3147. Microbiology 146:129–138 [DOI] [PubMed] [Google Scholar]
- 25. Moore D, Hamilton CM, Maneewannakul K, Mintz Y, Frost LS, Ippen-Ihler K. 1993. The Escherichia coli K-12 F plasmid gene traX is required for acetylation of F pilin. J. Bacteriol. 175:1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez RH, Himeno K, Ishibashi N, Masuda Y, Zendo T, Fujita K, Wilaipun P, Leelawatcharamas V, Nakayama J, Sonomoto K. 2012. Monitoring of the multiple bacteriocin production by Enterococcus faecium NKR-5-3 through a developed liquid chromatography and mass spectrometry-based quantification system. J. Biosci. Bioeng. 114:490–496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.