Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, must adapt to two diverse niches, an arthropod vector and a mammalian host. RpoS, an alternative sigma factor, plays a central role in spirochetal adaptation to the mammalian host by governing expression of many genes important for mammalian infection. B. burgdorferi is known to be unique in metal utilization, and little is known of the role of biologically available metals in B. burgdorferi. Here, we identified two transition metal ions, manganese (Mn2+) and zinc (Zn2+), that influenced regulation of RpoS. The intracellular Mn2+ level fluctuated approximately 20-fold under different conditions and inversely correlated with levels of RpoS and the major virulence factor OspC. Furthermore, an increase in intracellular Mn2+ repressed temperature-dependent induction of RpoS and OspC; this repression was overcome by an excess of Zn2+. Conversely, a decrease of intracellular Mn2+ by deletion of the Mn2+ transporter gene, bmtA, resulted in elevated levels of RpoS and OspC. Mn2+ affected RpoS through BosR, a Fur family homolog that is required for rpoS expression: elevated intracellular Mn2+ levels greatly reduced the level of BosR protein but not the level of bosR mRNA. Thus, Mn2+ and Zn2+ appeared to be important in modulation of the RpoS pathway that is essential to the life cycle of the Lyme disease spirochete. This finding supports the emerging notion that transition metals such as Mn2+ and Zn2+ play a critical role in regulation of virulence in bacteria.
INTRODUCTION
Borrelia burgdorferi is the causative agent of a multisystem infection of humans known as Lyme disease (1–3). B. burgdorferi is maintained in the enzootic cycle through a tick vector, Ixodes scapularis, and a mammalian host, e.g., the white-footed mouse, Peromyscus leucopus. B. burgdorferi can thrive in these two extremely diverse host environments by altering expression of its proteins such as outer surface protein A (OspA) and OspC (for a review, see references 4, 5, 6, and 7). OspA is produced chiefly in unfed ticks and is critical for spirochetal survival in the tick midgut environment (8–12), whereas OspC is produced during tick feeding and is critical for transmission and for the initial stage of mammalian infection (13–18).
It is well established that differential expression of OspC and many other mammalian infection-associated proteins are governed by the RpoN-RpoS pathway (or σ54-σS sigma factor cascade). In this pathway, an NtrC-like bacterial enhancer-binding protein (EBP), Rrp2, along with the alternative sigma factor RpoN (σ54 or σN), directly activates production of the other alternative sigma factor and global regulator RpoS (σS), through a −24/−12 σ54-type promoter sequence located upstream of the rpoS gene (19–25). In addition, rpoS can be transcribed to produce a long form of rpoS mRNA from an unknown “housekeeping” σ70-type promoter (26, 27). RpoS directly activates ospC (22) and functions as a global regulator that is indispensable for the enzootic cycle of B. burgdorferi (19, 20, 23, 24, 28).
Recent findings by two independent research groups on RpoS regulation show that BosR (Borrelia oxidative stress response regulator), a member of the ferric uptake regulator (Fur) family of transcriptional regulators, is essential for rpoS expression (29–34). BosR has been shown to bind to Zn2+ and may function as either an activator or a repressor for several genes involved in the oxidative stress response (29, 30, 35, 36). The requirement of BosR in rpoS activation is unexpected, since it is well established that in other bacteria, EBP and σ54 (along with the core RNA polymerase) are necessary and sufficient to activate transcription from a −24/−12 σ54-type promoter (37, 38). However, it has been reported that in other bacteria, Fur is involved in regulation of rpoS expression from a σ70-type promoter (39, 40). Recent DNA binding data imply a direct role of BosR in activation of the rpoS transcription through binding in the vicinity of the σ54-type promoter of rpoS (41). However, how BosR fits into the current dogma of σ54-dependent transcriptional activation remains to be elucidated.
Metals are vital cofactors for essential enzymes in biology. They are also important signals for gene regulation. For instance, the food-borne pathogen Salmonella enterica serovar Typhimurium alters the expression of virulence factors when magnesium or iron concentrations are low (42–45). Host immunobiology factors, such as the cytokine interleukin-6 (IL-6) and the regulators STAT-3 and SMAD-7, influence regulation of metal homeostasis (46, 47). Little is known about the role of biologically available metals on gene expression for B. burgdorferi. In this study, we showed that environmental signals that are known to affect the RpoN-RpoS pathway dramatically influenced the intracellular Mn2+ level, and an increase in the intracellular Mn2+ levels significantly repressed the RpoN-RpoS pathway, by reducing the BosR protein level via a yet-to-be identified mechanism. Mn2+ repression of BosR and RpoS could be overcome by excess Zn2+. Our findings support the notion that Mn2+ and Zn2+ have a critical role in regulation of virulence in bacteria (48).
MATERIALS AND METHODS
Bacterial strains.
B. burgdorferi strain 297 and the isogenic mutant bmtA were kindly provided by M. V. Norgard and Z. Ouyang (49), and strain B31-A3 was kindly provided by P. Rosa (50). For construction of the bmtA mutant in the B. burgdorferi strain B31-A3 background, 20 μg of the suicide plasmid DNA pOY04 (49) was transformed into B31-A3, and selection for mutants was performed as described previously (12, 51) with 500 μg/ml of kanamycin (Sigma-Aldrich, St. Louis, MO). One clone was obtained, and primers BbbmtA Fwd and BbbmtA Rev (Table 1) were used to confirm the mutation by PCR. The cultures used were no more than three passages from original stock (4), and spirochetes were grown using standard BSK-II medium (52) at 37°C in a 5% CO2 incubator.
Table 1.
Primers used in this study
| Namea | Sequence (5′ to 3′) |
|---|---|
| flaB fwd | ACCAGCATCACTTTCAGGGTCTCA |
| flaB rev | CAGCAATAGCTTCATCTTGGTTTG |
| bosR fwd | CATTTTATACATAGCATCAAACCC |
| bosR rev | TATATACTGTTGCTTTTGATAGGC |
| A3ospC fwd | TAGCGGGAGCTTATGCAATATCAACC |
| A3ospC rev | CATCAATTTTTTCCTTTAATCCTTCA |
| 297ospC fwd | AAAGGTGGGAATACATCTGC |
| 297ospC rev | TCTTTCACAGCCAGAACAAC |
| rpoS fwd | ATAAAAAGATATGCGGGTAAAGGG |
| rpoS rev | TGATTGCTTAATCCAAAATGATGC |
| bmtA fwd | TAATGGACGCTATGCTTGG |
| bmtA rev | AATGTATCCAAGCTCTTCAGC |
| BbbmtA Fwd | TTGTGGAGGCCCTCATGTAG |
| BbbmtA Rev | GAATATATCAGCGGAAAATTTGG |
Primers were ordered from IDT Integrated DNA Technologies.
Medium and growth conditions.
BSK-II medium supplemented with 6% rabbit serum was used throughout (53). To reduce the divalent cations in BSK-II medium, Chelex 100 (Bio-Rad, Hercules, CA) was used to treat the medium as previously described (54). Briefly, BSK-II medium was prepared, and 50 g/liter of Chelex 100 resin was added to the medium followed by gentle stirring at 4°C for 1 h. The Chelex-treated medium was centrifuged at 7,000 × g for 30 min, the pH of the supernatant was reduced to 7.5 or 7.0 by the addition of HCl, and then the mixture was sterilized by filtration. This process removed any remaining Chelex 100 resin from the medium. Metal analysis by inductively coupled plasma mass spectrometry (ICP-MS) confirmed that Chelex treatment reduced manganese concentration to below detection. Medium was stored at −80°C until use.
Cultures from −80°C storage were inoculated into BSK-II medium and cultivated at 37°C in a 5% CO2 incubator until the exponential phase (∼1 × 107 cells/ml). Then, cultures were diluted to 105 cells/ml for experiments. Cultures were grown at 37°C for 5 to 7 days, washed with 0.9% NaCl, and treated for denaturing and reducing SDS-PAGE. For additional experiments, cultures were grown at 25°C for 21 days.
SDS-PAGE and immunoblotting.
Denatured and reduced samples prepared as described above were resuspended in Laemmli sample buffer (Bio-Rad, Hercules, CA) and boiled. Denatured proteins were separated on Mini-Protean TGX gels (12% acrylamide; Bio-Rad) and transferred to 0.45-μm nitrocellulose membranes (Bio-Rad). Transfer was confirmed by Ponceau S staining of the membrane. Primary antibodies against FlaB, RpoS, and OspC were reported previously (32, 55, 56). The antibody against BosR used in this study was purchased from General Bioscience Corp. (Brisbane, CA). Secondary antibody (peroxidase-conjugated goat anti-mouse antibody; Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:1,000. Detection of horseradish peroxidase activity was determined using 4-chloro-1-naphthol and H2O2 (Fisher Scientific).
Metal analysis by ICP-MS.
To measure intracellular metal content, strains were grown in BSK-II or Chelex-treated BSK-II medium at 37°C for 7 days (initial cell density, 105 cells/ml). Samples (n ≥ 3) were centrifuged, washed two times in phosphate–100 μM EDTA buffer (pH 7.8), and concentrated 100-fold in buffer. Samples were placed in a drying oven at 95°C for 24 h, and a dry weight measurement was recorded. Dry cell pellets were resuspended in 3 N nitric acid and heated in a drying oven as described above. Control samples without bacteria that included the wash buffer and acid treatments were included with each analysis to account for the presence of metals in the buffer reagents, on the surface of lab equipment, etc. Values of metal content obtained with the control samples were subtracted from the values recorded from biological samples. Acid-treated samples were resuspended in 0.5 ml of 3 N nitric acid and sent for analysis at the Analytical Spectroscopy Services Laboratory (ASSL) located at North Carolina State University and analyzed using a Varian 820 ICP-MS.
qRT-PCR.
Primers used in quantitative reverse transcriptase PCR (qRT-PCR) are listed in Table 1. To determine the change in transcription of target genes (flaB, bmtA, rpoS, bosR, and ospC), strains were grown to the stationary phase and samples were split into separate tubes for Western blotting and RNA purification. Total RNA was isolated using TRIzol reagent (Invitrogen). Following purification, total RNA was treated with DNase I (New England BioLabs) for 1 h at 37°C. Then, the DNase-treated RNA was purified using the RNA cleanup protocol with the RNeasy miniprep kit (Qiagen). cDNA was synthesized as follows: 1 μl of a 10 mM deoxynucleoside triphosphate (dNTP) mixture (2.5 mM each dNTP), 1 μl of 50 μM gene-specific primer, total RNA, and H2O were added to a final volume of 13 μl. The sample was heated at 65°C for 5 min and then placed on ice for 1 min. Then, 5 μl of 5× first-strand synthesis buffer (Invitrogen), 1 μl of 0.1 M dithiothreitol (DTT; Invitrogen), 1 μl of RNase OUT (Invitrogen), and 1 μl of Superscript reverse transcriptase III (Invitrogen) were added. The sample was incubated at room temperature for 5 min and then at 50°C for 60 min and at 70°C for 15 min. Following cDNA synthesis, 20 or 30 μl of double-distilled water (ddH2O) was added to the sample. A control receiving no reverse transcriptase was included for each RNA sample.
qRT-PCR was performed, using the RT2 SYBR green ROX qPCR Mastermix, as follows: 10 μl of qPCR Mastermix, 0.5 μM primers, 2 μl of cDNA, and ddH2O to a 20-μl final volume were added to a 96-well plate (Applied Biosystems). An ABI Prism 7000 real-time PCR machine was used with the following PCR parameters: 95°C for 10 min with 40 cycles of 95°C for 15 s, 50°C for 30 s, and 72°C for 30 s. Melting curve analysis confirmed the presence of a single PCR product for each sample. A standard curve of flaB DNA (102 to 107 copies per reaction mixture) was used to quantify transcripts, and expression data were normalized per 1,000 copies of flaB. qPCR with no reverse transcriptase control, targeting flaB, confirmed the reduction of ≥5 orders of magnitude in copy number in the samples. These results indicated that genomic DNA contamination was near background level.
Statistical analyses.
Statistical significance was determined using Student's t test, and when multiple comparisons were made, the P value was corrected using the Bonferroni correction. One-way analysis of variance (ANOVA) was used to determine significance, and results are shown in Table 2.
Table 2.
Exogenous addition of Mn2+ and Zn2+ increases the intracellular Mn2+ and Zn2+ concentrationsa
| Medium | Mean intracellular metal content ± SD (μmol/g, dry wt) |
||
|---|---|---|---|
| Mn2+ | Zn2+ | Mn2+/Zn2+ ratio | |
| BSK-II | 0.18 ± 0.08 | 0.93 ± 0.14 | 1:5 |
| BSK-II Chelex | 0.03 ± 0.01 | 0.60 ± 0.02 | 1:20 |
| + 10 μM MnCl2 | 0.53 ± 0.19 | 0.45 ± 0.06 | 1 |
| + 10 μM ZnSO4 | 0.04 ± 0.02 | 1.20 ± 0.11 | 1:30 |
| + 100 μM ZnSO4 | 0.01 ± 0.01 | 1.82 ± 0.06 | 1:182 |
| + 10 μM MnCl2 + 10 μM ZnSO4 | 0.69 ± 0.16 | 0.68 ± 0.21 | 1 |
| +10 μM MnCl2 + 100 μM ZnSO4 | 0.35 ± 0.10 | 1.36 ± 0.19 | 1:4 |
B. burgdorferi strain 297 was cultivated at 37°C to stationary phase, and intracellular metal contents from two separate experiments with three independent cultures were determined by ICP-MS. Bold values are significantly different from those of the corresponding metal from Chelex-treated BSK-II (P < 0.05). One-way ANOVA with Dunnett's multiple-comparisons test was used to determine significance.
RESULTS
Intracellular Mn2+ inversely correlates with OspC expression.
To investigate whether intracellular metals may influence expression of virulence factors in B. burgdorferi, we analyzed the intracellular metal content under various growth conditions. It is well established that elevated temperature induces many virulence factors such as ospC via direct activation by RpoS (13, 23, 26, 57–59). Thus, a culture growing at 37°C (at late log or stationary phase) has been considered to be under an RpoS “on” condition, whereas a culture incubated at 25°C is regarded as being under an RpoS “off” condition (Fig. 1A). We also examined the level of BosR, because in addition to RpoN and Rrp2, BosR was recently shown to be required for the transcriptional activation of rpoS (19, 32, 41, 60). As shown in Fig. 1A, BosR was also induced by elevated temperature, suggesting that temperature-induced rpoS activation is, at least in part, through BosR. We then examined the intracellular concentrations of two transition metal ions, Mn2+ and Zn2+, in B. burgdorferi strain 297 cultivated under either 25°C or 37°C conditions. The results showed no significant difference in the intracellular Zn2+ levels between 25°C and 37°C samples (Fig. 1B, right panel). Surprisingly, we detected a 5-fold-lower intracellular Mn2+ level when spirochetes were cultivated at 37°C compared to 25°C (Fig. 1B, left panel). At 25°C, the intracellular Zn2+ and Mn2+ levels were similar, whereas at 37°C, the level of Mn2+ was more than 6-fold lower than the level of Zn2+. In other words, the intracellular ratio of Mn2+ to Zn2+ was ∼1:1 at 25°C and ∼1:6 at 37°C. These results suggest that intracellular Mn2+ is temperature regulated and inversely correlates with RpoS activation.
Fig 1.
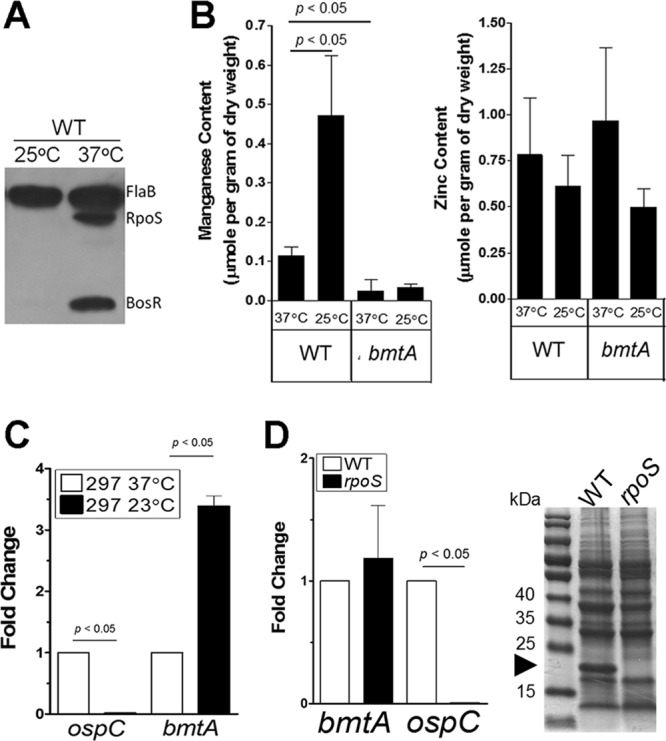
Intracellular manganese content inversely correlates with temperature-dependent activation of RpoS and OspC. (A) Immunoblot analysis demonstrating an RpoS “on” condition when wild-type B. burgdorferi strain 297 was cultivated at 37°C and an RpoS “off” condition when cultivated at 25°C. The level of BosR correlated well with expression of RpoS. The constitutive active FlaB serves as a control. Results are representative of two separate experiments with 3 replicates per experiment. (B) Metal analysis by inductively coupled plasma mass spectrometry (ICP-MS). The parental strain 297 (WT) and the isogenic bmtA mutant were grown in standard BSK-II medium at 37°C or at 25°C. Samples were harvested at the stationary phase and analyzed for intracellular Mn2+ (left panel) and Zn2+ (right panel). Data represent the means ± standard deviations (SD) of 3 independent samples from two separate experiments. (C) Expression of bmtA is temperature dependent. Wild-type B. burgdorferi strain 297 was grown in standard BSK-II medium at 37°C or 25°C and harvested at the stationary phase. RNAs were extracted from 3 independent cultures, and expressions of flaB, ospC, and bmtA were determined by qRT-PCR. Data were normalized to 1,000 copies of flaB, and the fold changes are shown with expressions at 37°C set to 1. Significance was determined by Student's t test. (D) Expression of bmtA is RpoS independent. Wild-type B. burgdorferi 297 and the isogenic rpoS mutant were grown in standard BSK-II medium at 37°C and harvested at the stationary phase. Expressions of flaB, ospC, and bmtA were determined by qRT-PCR from total RNA extracted from 3 independent cultures. Cell lysates were separated by SDS-PAGE, and a representative Coomassie blue-stained gel is shown in the right panel. The arrowhead indicates the band corresponding to OspC.
To validate the measurement of intracellular metal concentrations, a bmtA mutant of strain 297 that was previously reported by Ouyang et al. (49) was included in the experiments. BmtA is involved in metal homeostasis by functioning as an Mn2+ transporter in B. burgdorferi (49). Consistent with the previous finding, the bmtA mutant exhibited 12-fold reduction of intracellular Mn2+ compared to the parental strain 297 grown at 37°C (Fig. 1B) (49). However, in contrast to wild-type spirochetes, the bmtA mutant cultivated at 25°C had low intracellular Mn2+ levels (Fig. 1B), suggesting that the increased level of Mn2+ observed in wild-type spirochetes at 25°C is BmtA dependent.
Culture temperature influences expression of bmtA.
Because the increased intracellular Mn2+ content at 25°C was BmtA dependent, we further examined the influence of culture temperature on bmtA expression. As expected, the expression of ospC at 25°C was reduced >200-fold compared to that at 37°C (Fig. 1C). Under the same conditions, bmtA expression was increased >3-fold by cultivation at 25°C compared to 37°C (Fig. 1C). This is consistent with previously published microarray data (61). Thus, expression of bmtA appears to be temperature regulated, which contributes to the increased Mn2+ level by lowered temperature.
Since the level of RpoS is regulated by culture temperature, we examined the possibility that RpoS may negatively regulate bmtA transcription. To test this, wild-type B. burgdorferi strain 297 and the isogenic rpoS mutant were grown to stationary phase, and protein and RNA were prepared from each biological replicate. As shown previously, wild-type B. burgdorferi produced OspC that can be easily detected in Coomassie blue-stained gels under the optimal conditions (37°C, stationary phase) (26, 28, 55, 56, 62, 63) (Fig. 1D, right panel). Inactivation of rpoS abolished ospC expression at both RNA and protein levels (Fig. 1D) (19, 64). However, the expression of bmtA was virtually unchanged in the rpoS mutant (Fig. 1D, left panel). Thus, the regulation of bmtA appears to be independent of RpoS. These results suggest that the increased bmtA expression and the Mn2+ level at 25°C were not the result of downregulation of RpoS. Rather, the increased Mn2+ level at 25°C may negatively regulate RpoS levels.
Mn2+ negatively regulates OspC and RpoS but is overcome by excess Zn2+.
The inverse correlation of the intracellular Mn2+ levels with RpoS levels suggests that Mn2+ may negatively regulate RpoS. If so, an increase in intracellular Mn2+ level should inhibit RpoS and OspC production, even in spirochetes grown under RpoS “on” conditions (37°C). To address this, manipulation of the metal status in the complex BSK-II medium is required. To this end, we treated standard BSK-II medium with Chelex-100 resin to chelate divalent cations from the medium. We determined the effectiveness of the Chelex-100 treatment by measuring Mn2+ and Zn2+ levels before and after treatment. BSK-II medium contains a low concentration of Mn2+, which can be reduced to an undetectable concentration (<0.5 μg/liter, ∼10 nM) upon treatment with Chelex-100 resin (Fig. 2A). Furthermore, Zn2+, which is substantially higher than Mn2+ in BSK-II, can be reduced 6-fold by Chelex treatment (Fig. 2B). Chelex treatment did not completely remove all the metals, but the dramatic reduction of Mn2+ allows us to investigate the potential role of Mn2+ in RpoS and OspC activation.
Fig 2.
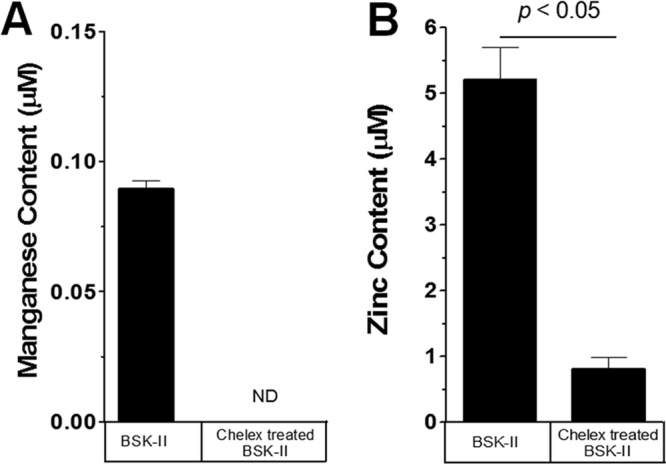
Manganese and zinc in BSK-II medium can be reduced by Chelex treatment. The manganese (A) and zinc (B) contents of BSK-II medium and Chelex-treated BSK-II medium were measured as described in Materials and Methods. ND, not detected. Data are from three separate batches of Chelex-treated BSK-II. Significance was determined by Student's t test.
B. burgdorferi strain 297 grown in Chelex-treated or untreated BSK-II medium exhibited no discernible differences at 37°C in either growth rate or final cell density (Fig. 3A), despite virtually no detectable amount of Mn2+ in the medium. This was not surprising, given that the bmtA mutant showed little effect on cell growth (49). However, the level of OspC was enhanced when spirochetes were cultivated in the Chelex-treated BSK-II medium (Fig. 3B). This result implied that cultivation of spirochetes in an Mn-limited medium enhanced OspC levels. To determine whether this enhanced OspC production with Chelex-treated medium was due to the reduction of Mn2+ rather than to a reduction of other divalent cations, Chelex-treated BSK-II was then supplemented with various concentrations of MnCl2 or ZnSO4. As shown in Fig. 3C, addition of 10 μM MnCl2 to this medium dramatically reduced the production of both RpoS and OspC (Fig. 3C, second lane from left), but addition of 10 μM ZnSO4 did not reduce production of OspC (Fig. 3C, third lane from left). These results suggested that Mn2+, not Zn2+, played a repressive role in the production of OspC and RpoS.
Fig 3.
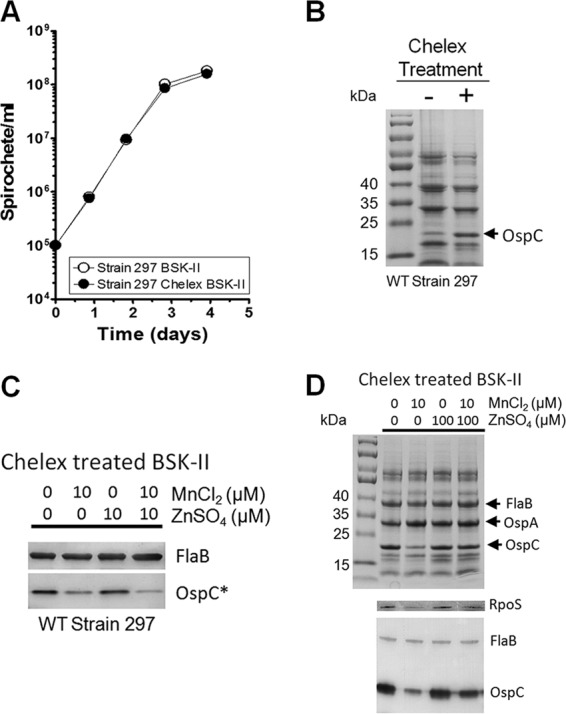
Influence of RpoS and OspC production by Mn2+ and Zn2+ in B. burgdorferi strain 297. (A) Growth curves of wild-type B. burgdorferi strain 297 in standard or Chelex-treated BSK-II medium. The means with SD from two separate experiments are shown with duplicates of each sample. (B) Protein profiles of spirochetes grown in standard or Chelex-treated medium. Cells were harvested at the late log phase, and cell lysates were subjected to SDS-PAGE analysis. The bands corresponding to OspC are indicated by an arrow. Results are representative of the two separate experiments. (C and D) Effects of Mn2+ and Zn2+on RpoS and OspC levels. Strain 297 was grown at 37°C in Chelex-treated BSK-II medium (pH 7.5) with or without added 10 μM MnCl2 and/or ZnSO4 (C) or 100 μM ZnSO4 (D). Equivalent amounts of protein were separated by SDS-PAGE (D, top panel) and immunoblotted with antibodies against FlaB, RpoS, or OspC (C and D, bottom panels). FlaB served as a loading control. *, a 10-fold dilution of each sample was used in this immunoblot assay to avoid saturation of the OspC signal. Data shown in panels C and D are representative from two separate experiments performed with samples in triplicate.
We further investigated whether Zn2+ would influence the effect of Mn2+ by supplementing both ZnSO4 and MnCl2 to the Chelex-treated medium. The result showed that reduced RpoS and OspC production by Mn2+ could not be rescued or enhanced by addition of equimolar amount of Zn2+ (Fig. 3C, fourth lane from the left). However, when a 10-fold excess of ZnSO4 (100 μM) to MnCl2 (10 μM) was added, the production of RpoS and OspC was restored (Fig. 3D, fifth lane from left). This result suggested that Zn2+ played a positive role in RpoS production and overcame the inhibitory effect of Mn2+.
Further analyses confirmed that the intracellular Mn2+ and Zn2+ levels were correlated positively with exogenous availability of Mn2+ and Zn2+ (Table 2). When grown in Chelex BSK-II medium, the intracellular Mn2+ concentration was dramatically reduced compared to that observed in growth in BSK-II medium (Table 2), which confirmed that this medium is Mn limited compared to BSK-II. Supplementing Chelex BSK-II medium with 10 μM MnCl2 increased the intracellular Mn2+ level of B. burgdorferi 20-fold in comparison to that of spirochetes grown in Chelex BSK-II medium (Table 2). In contrast to Mn2+, intracellular Zn2+ levels were moderately affected (3-fold increase upon supplementation with 100 μM ZnSO4). This result further suggests that intracellular Mn2+ concentration is highly sensitive to environmental Mn2+ levels and that Mn2+ may serve as an environmental sensor for modulating gene expression.
The above-described experiments were conducted with the infectious B. burgdorferi strain 297. Since it is known that different strains of B. burgdorferi express various levels of RpoS and OspC (64), the influence of Mn2+ and Zn2+ on RpoS and OspC was further confirmed using B. burgdorferi strain B31-A3 (50). Because we found that B31-A3 often expresses a low level of RpoS and OspC relative to strain 297 when cultivated under the standard conditions (BSK-II medium, pH 7.5, 37°C), we therefore cultivated B. burgdorferi strain B31-A3 in Chelex-treated BSK-II that was adjusted to pH 7.0, since reduced culture pH enhances RpoS and OspC expression of B. burgdorferi (56, 65). As shown in Fig. 4, OspC induction was readily observed on the Coomassie blue-stained gel in strain B31-A3 grown under such conditions (Fig. 4A). Similar to what was observed for strain 297, addition of MnCl2 to the medium inhibited production of OspC (Fig. 4A) and RpoS (Fig. 4B) in strain B31-A3, suggesting that the repressive effect of Mn2+ on RpoS is not strain dependent. The positive effect of Zn2+ on RpoS production was stronger in strain B31-A3 than that in strain 297: excess Zn2+ (100 μM) not only was able to overcome the inhibitory effect of Mn2+ on RpoS but also further enhanced OspC production, in the absence or presence of Mn2+ (Fig. 4A, fourth and fifth lanes from left). These results provide further evidence that Mn2+ exhibits a repressive role in RpoS production, whereas Zn2+ exerts a positive role.
Fig 4.
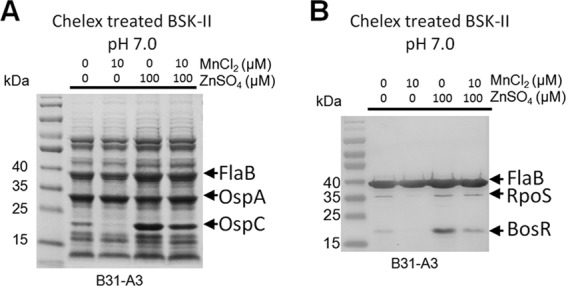
Influence of Mn2+ and Zn2+ on RpoS and OspC production in B. burgdorferi strain B31. Strain B31-A3 was grown at 37°C in Chelex-treated BSK-II medium adjusted to pH 7.0, with addition of various amounts of Mn2+ and/or Zn2+. Samples were harvested at the late logarithmic phase, and cell lysates were detected by Coomassie blue-stained SDS-PAGE gel (A) or by immunoblotting using antisera against FlaB, RpoS, and BosR (B). Data are representative of two separate experiments performed in triplicate.
Mn2+ negatively regulates RpoS through BosR.
It is well established that expression of rpoS is governed by two transcriptional factors, Rrp2 and BosR (Fig. 1A) (31, 32, 60). Since BosR is a Zn-dependent transcriptional activator of rpoS, we focused on BosR (29). We cultivated strain B31-A3 in Chelex-treated BSK-II at pH 7.0 with and without the addition of metals. Addition of MnCl2 suppressed not only OspC and RpoS but also the BosR level (Fig. 4B). Similarly, excess Zn2+ also enhanced the level of BosR (Fig. 4). Given that BosR is an essential activator for rpoS, we conclude that the molecular mechanism for metal-dependent regulation of RpoS is through affecting the level of BosR.
Deletion of bmtA enhances BosR level.
To gather further genetic evidence supporting the finding that Mn2+ suppresses BosR, we examined the bmtA mutant that was previously generated in strain 297 by Ouyang et al. (49). BmtA is an Mn2+ transporter in B. burgdorferi; deletion of bmtA resulted in reduced intracellular levels of Mn2+ (49) (Fig. 1). If Mn2+ plays a negative role in the RpoS pathway via BosR, deletion of bmtA should lead to elevated OspC and BosR levels. Indeed, when the spirochetes lacking BmtA were grown in BSK-II medium at 37°C, the ΔbmtA strain had higher levels of OspC and BosR than did parental and complemented strains when harvested at the same cell density (late log phase) (Fig. 5A). We confirmed that the ΔbmtA mutant had increased ospC promoter activity by measuring the β-galactosidase activity in a ΔbmtA strain harboring a previously described shuttle vector that encodes an ospC promoter fused with the lacZ reporter (pBHospCp-lacZBb) (data not shown). It is known that elevated pH (pH 8) inhibits the activation of the RpoS pathway in wild-type B. burgdorferi (56, 65). However, the bmtA mutant was even able to overcome the inhibitory effect by pH 8 and produced OspC (data not shown). On the other hand, the bmtA mutant remained incapable of expressing OspC when cultivated at 25°C despite the low intracellular Mn2+ level (data not shown), suggesting that some additional factor(s) was required for activating the pathway at lower temperature. Nevertheless, these results indicate that deletion of bmtA resulted in enhanced activation of the RpoS pathway, and this effect was through an increased BosR level.
Fig 5.
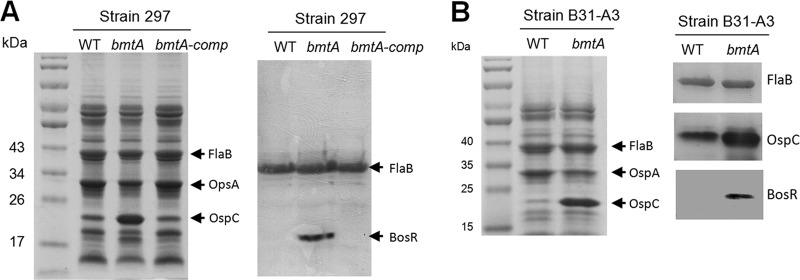
Deletion of bmtA resulted in elevated OspC and BosR production. Wild-type B. burgdorferi strain 297 (A) or strain B31-A3 (B) and the isogenic bmtA mutant or the complemented strain (bmtA-comp) were grown in BSK-II medium (pH 7.5) at 37°C and harvested at the late logarithmic phase. Equal amounts of whole-cell lysates were separated by Coomassie blue-stained SDS-PAGE gel (left panels), or probed for FlaB or BosR protein by immunoblotting analyses (right panels). Data shown are representative of two separate experiments performed in duplicate.
To further confirm that inactivation of bmtA enhanced BosR and RpoS levels, we constructed a new bmtA mutant in B. burgdorferi strain B31-A3 (see Fig. S1A in the supplemental material). Deletion of bmtA in B31-A3 did not alter the growth kinetics compared to the parental strain (see Fig. S1B in the supplemental material). However, similar to the bmtA mutant in the strain 297 background, the bmtA mutant in B31-A3 showed dramatic upregulation of OspC and BosR levels compared to its parental strain (Fig. 5B). These combined data provided strong genetic evidence to support the conclusion that Mn2+ negatively regulates the RpoS pathway through influencing the level of BosR.
Mn2+ represses BosR at the posttranscriptional level.
To gain insight into how metals may affect BosR levels, we investigated whether BosR is regulated at the transcriptional or posttranscriptional level. First, we compared the BosR protein level and mRNA level upon addition of Mn2+. Strain B31-A3 was cultivated in Chelex-treated BSK-II at pH 7.0 with or without added MnCl2. Spirochetes were harvested at stationary phase, and expression levels of bosR and rpoS mRNA or their proteins were determined by qRT-PCR and immunoblotting. As shown in Fig. 6A, Mn2+ dramatically reduced rpoS expression at both mRNA and protein levels. However, although the BosR protein level was dramatically reduced, the bosR mRNA level was not significantly influenced by the addition of MnCl2 (Fig. 6A). We also compared the protein and RNA levels of BosR in both of the bmtA mutants generated in the 297 and B31-A3 backgrounds. Both bmtA mutants showed dramatic increases in the BosR protein level but little change in the bosR mRNA level (Fig. 6B and C). Taken together, we conclude that Mn2+ suppresses BosR expression at the posttranscriptional level.
Fig 6.
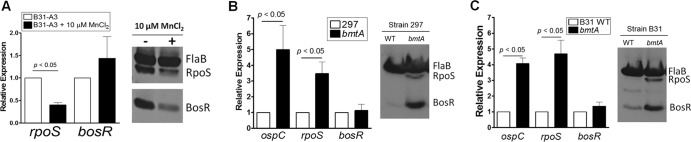
Manganese downregulates BosR at the posttranscriptional level. (A) Addition of MnCl2 downregulates BosR at the posttranscriptional level. Strain B31-A3 was grown at 37°C in Chelex-treated BSK-II medium (pH 7.0) with or without addition of 10 μM MnCl2. Spirochetes were harvested at the late log phase and subjected to qRT-PCR (left panel) and immunoblotting analyses (right panel). qRT-PCR data are from 3 independent samples with significance determined by Student's t test. A representative result from immunoblotting of samples is shown. (B and C) The bmtA mutant in a 297 background (B) or a B31 background (C) enhances BosR protein at the posttranscriptional level. The parental strains and the isogenic bmtA mutant were grown in BSK-II medium (pH 7.5) at 37°C and harvested at the late logarithmic phase. Spirochetes were then subjected to qRT-PCR (left panel) and representative immunoblotting analyses (right panel) as described for panel A.
DISCUSSION
Metalloenzymes are essential for biological systems, and the metal status is tightly regulated within the cell. Many bacteria encode metal-dependent transcription factors that sense the intracellular metal status and regulate gene expression in response to stimuli. Fur is one of the most studied metal-dependent transcription factors regulating metal homeostasis and virulence (66, 67). Very little is known about the function and regulation of metals in the Lyme disease spirochete. In this study, we demonstrated that two transition metal ions, Mn2+ and Zn2+, were important in the regulation of RpoS, a central regulator that governs differential expression of many virulence genes in B. burgdorferi (19, 20, 23, 24, 26, 28). We showed that Mn2+ suppression of RpoS was through the Fur family homolog BosR, an essential activator for rpoS transcription. Furthermore, we demonstrate that Mn2+ influences BosR at its protein level. This is the first report that any biologically relevant metal ion influences virulence factor expression in B. burgdorferi.
Several lines of evidence support the conclusion that the intracellular Mn2+ concentration is dramatically regulated and controls the transcription of rpoS via BosR. First, cultivation of spirochetes at 25°C dramatically increased the intracellular Mn2+ concentration of B. burgdorferi (Fig. 1). Second, addition of exogenous Mn2+ increased the intracellular Mn2+ (Table 2). Such a dramatic change in intracellular Mn2+ levels suggests that Mn2+ can function as an environmental sensor. Third, the increased intracellular Mn2+ dramatically repressed transcription of temperature and pH-induced rpoS and ospC (Fig. 3, 4, and 6). Such repression could be relieved by addition of excess Zn2+. Likewise, a biochemical approach consisting of reducing the Mn2+ content of BSK-II with Chelex treatment enhanced OspC production in wild-type bacteria (Fig. 3A). Finally, we provided genetic evidence that the bmtA mutant with decreased intracellular Mn2+ had enhanced transcription of rpoS and ospC in two strain backgrounds (Fig. 5 and 6). Thus, Mn2+ appears to exert a negative regulation on RpoS, via negative regulation of BosR. Together with our previous finding that Mn2+ exerts positive regulation on SodA, an Mn2+-dependent superoxide dismutase (SOD) of B. burgdorferi (54, 68), we postulate that Mn2+ inversely regulates the levels of BosR and SodA and plays an important role in regulating virulence factors of B. burgdorferi as summarized in the model (Fig. 7).
Fig 7.
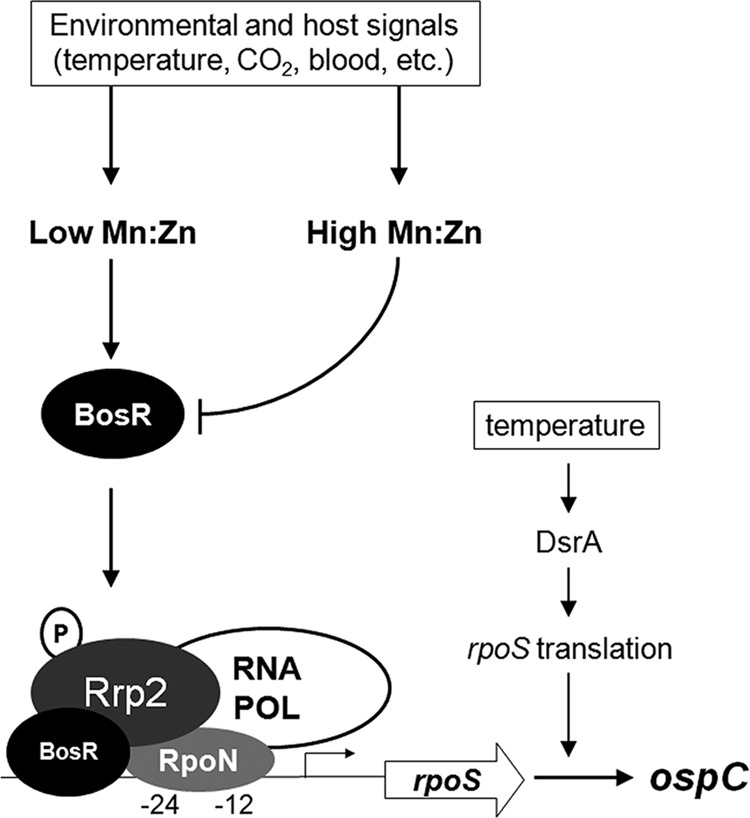
Model depicting the role of Mn2+ and Zn2+ in the regulation of the RpoS pathway through BosR. Expression of rpoS is modulated by two transcriptional factors, Rrp2 and BosR. Rrp2 activity is modulated via phosphorylation by acetyl∼P or Hk2 (80, 99). For the purpose of simplification, factors that influence RpoS through acetyl∼P such as CsrA, OppA5, acetate, and the mevalonate pathway are not included in the model (63, 80, 84–86). Environmental and host signals, such as temperature and blood, can modulate rpoS expression by altering the intracellular Mn2+ and Zn2+ status and subsequently BosR levels. A low intracellular Mn2+ and high Zn2+ status promotes a high level of BosR protein (not bosR transcript), leading to the increased level of RpoS and RpoS-controlled products such as OspC. In addition, temperature can also regulate RpoS levels via small RNA DsrA at low cell density (26).
The dramatic changes of intracellular Mn2+ within B. burgdorferi observed in this study are not unique among bacterial pathogens. The intracellular Mn2+ content is very low under routine culture conditions for Escherichia coli and S. Typhimurium but increases dramatically under specific conditions (69, 70). In these organisms, repression of Mn2+ transport is accomplished by redox-sensing transcription factors, mainly DtxR or Fur homologs, which promote Mn2+ uptake under conditions of oxidative stress (1, 69, 70). Our metal analysis with ΔbosR in B. burgdorferi did not reveal enhanced intracellular concentration of Mn2+ (data not shown), suggesting that regulation of Mn2+ transport in B. burgdorferi is BosR independent. Thus, the regulation of Mn2+ transport in B. burgdorferi appeared to be distinct from that of other bacterial pathogens. Regarding Zn2+, the relatively constant intracellular Zn2+ levels within B. burgdorferi (Table 2) are also consistent with the tightly regulated intracellular Zn2+ in other bacteria (71–76). Expression of Zn2+ transporters is typically repressed by the Fur homolog Zinc uptake regulator (Zur) or by family members of the Fur-like protein DtxR. Other related pathogenic spirochetes, Treponema pallidum and Treponema denticola, encode a DtxR protein called TroR (77, 78), which utilizes both Mn2+ and Zn2+ as corepressors (78, 79). BLASTP analysis within B. burgdorferi failed to identify a TroR homolog, indicating that regulation of Zn2+ transport within this spirochete may be unique. Interestingly, the finding in this study suggests that there is a connection between the intracellular concentration and transport of Mn2+ and Zn2+ (Table 2).
Temperature is one of the important environmental cues that induce RpoS and OspC production in B. burgdorferi (6, 7, 13). Previously, the small RNA DsrA was shown to link temperature sensing to RpoS production, by controlling the translation of the long form of the rpoS transcript (derived from a non-σ54-type promoter at low cell density) (26) (Fig. 7). How temperature induces rpoS transcription from the σ54(RpoN)-type promoter (the short and major form of rpoS transcript) remains unclear. It is known, however, that this RpoN-dependent transcription of rpoS requires Rrp2 and BosR (19, 32, 41, 60). It has been proposed that phosphorylation of Rrp2 is the signal that activates the RpoN-dependent transcription (60, 80), but whether temperature influences Rrp2 phosphorylation and subsequently controls rpoS transcription has not been tested, largely due to the lack of a method to detect Rrp2 phosphorylation in the cell. Nevertheless, we showed in this study that BosR protein was dramatically induced by elevated temperature (Fig. 1A). This observation indicates that temperature influences the RpoS regulon, at least in part, through regulation of the level of BosR. Thus, temperature influences the level of RpoS in at least two ways (Fig. 7): one is σ54 (RpoN) independent at low cell density, through DsrA; the other is σ54 (RpoN) dependent, through altering the intracellular Mn2+ and Zn2+ levels and subsequently the BosR level. What is the physiological significance of increased Mn2+ concentration within B. burgdorferi when cultivated at lower temperature (25°C)? We speculate that the increased solubility of O2 in water at 25°C compared to 37°C may lead to an increased formation of O2−, and increased intracellular Mn2+ concentration would enhance the expression of SodA to protect against O2− stress at lower temperature. An increase in the cytosolic levels of Mn2+ and/or Zn2+ has been reported in other bacteria in response to reactive oxygen stress (81).
In addition to temperature, many environmental factors, such as cell density, pH, CO2, and host blood, have been shown to influence RpoS levels (13, 35, 56, 61, 65, 82, 83). However, how these multiple factors collectively influence the rpoS expression has not been fully elucidated. We previously showed that multiple environmental factors may influence the intracellular concentration of acetyl∼P, a high-energy small phosphate important for phosphorylation and activation of Rrp2 (80). Subsequently, several studies showed factors or pathways that influence RpoS through acetate and acetyl∼P, including CsrA, OppA5, and the mevalonate pathway (63, 84–86). More recently, Jutras et al. proposed that the temperature effect on RpoS actually is due to the change of cell growth rate (100). Given that it is known that increase in growth rate leads to enhanced levels of acetyl∼P (87, 88), the effect of growth rate on RpoS is likely through acetyl∼P. Based on the findings in this study, we hypothesize that these environmental cues may also collectively influence the intracellular Mn2+ and Zn2+ levels and further affect RpoS levels. For cell density, although the growth phase regulation of Mn transport was not examined in this study, it has been reported that in other organisms Mn2+ transport genes are upregulated during transition from the lag phase to the exponential phase of growth (89). This may explain why induction of RpoS requires both elevated temperature and increased cell density (56). For pH, lowered pH (pH 7) enhanced RpoS and OspC expression (13, 56, 65). We showed that addition of Mn2+ was capable of inhibiting pH-induced BosR and RpoS activation (Fig. 4). The effect of mammalian blood on rpoS expression in feeding ticks may also function via Mn2+ and Zn2+ levels. When blood is added to BSK-II medium, RpoS-activated genes are induced (82). The high concentration of Zn2+ relative to low concentration of Mn2+ in human blood (which contains about 100 μM Zn2+ [90–92]) may account for this observation. Increased CO2 has been shown to enhance BosR, RpoS, and OspC levels (83). We postulate that increased CO2 may suppress Mn2+ transport and further lead to reduced intracellular Mn2+ level. It is known that Mn2+ import is influenced by dissolved gases such as O2 and during oxidative stress (69, 93). Thus, Mn2+ transport in B. burgdorferi may be negatively regulated by CO2, which further increases the level of BosR. This prediction is supported by evidence from other bacterial pathogens whose genes responsible for Mn2+ transport are repressed by metal-dependent transcription factors under anaerobic conditions (70, 93).
Little is known about metal utilization in B. burgdorferi. It was reported that B. burgdorferi does not require or transport iron (94). Interestingly, a recent report by Wang et al. showed that B. burgdorferi contains both iron and copper (62). Our data in this study and previous studies by others (49, 94) indicate that Zn2+ is a substantial metal within the Lyme disease spirochete. The precise role of Zn2+ in the physiology of this pathogen is not completely understood; however, its abundance suggests that Zn2+ may be essential. In addition to BosR, other possible Zn2+-binding proteins have been identified in B. burgdorferi, including NapA (BB0690), the peptide deformylase (BB0065), and the glycolytic enzyme fructose-1,6-bisphosphate aldolase (BB0445) (95, 96). Both BB00065 and BB0445 are likely essential genes. Mn2+ is present at lower concentrations than Zn2+ in Borrelia when replicating at 37°C, suggesting a limited number of Mn2+-dependent proteins within B. burgdorferi. The ability to delete the Mn2+ transporter, bmtA, and the fact that the ΔbmtA mutant can grow in BSK-II medium as well as within dialysis membranes implanted in rats (49) suggest that Mn2+ may not be a prominent cofactor for B. burgdorferi physiology. Moreover, the ΔbmtA mutant's growth was indistinguishable from that of the B31-A3 parent strain in Chelex-treated BSK-II (see Fig. S1C in the supplemental material). However, despite a 12-fold reduction, we were able to detect a low concentration of Mn2+ within the ΔbmtA strain (Fig. 1). The concentration of Mn2+ detected in the ΔbmtA mutant was >2- to 3-fold above the Mn2+ present within the wash buffer, indicating that Mn2+ values from the ΔbmtA mutant were well above background. Thus, the essentiality of Mn2+ cannot be ruled out. For example, the B. burgdorferi genome carries genes econding two likely essential glycolytic enzymes that may require Mn2+ as cofactors, including BB0004, encoding a putative phosphoglucomutase (EC 5.4.2.2) that catalyzes the conversion of glucose-1-phosphate to glucose-6-phosphate (97), and BB0658, encoding a putative phosphoglycerate mutase (Pgm; EC 5.4.2.1) (98). The fact that the ΔbmtA mutant is unable to infect mice or colonize ticks (49) supports the theory that Mn2+ may be essential in these Mn2+-limiting environments.
What is the mechanism underlying the negative regulation of BosR by Mn2+? Regulation of BosR by CO2 has been previously observed at the posttranscriptional level (30, 61, 83). In this study, we showed that Mn2+ suppressed bosR at the posttranscriptional level, i.e., that the bosR mRNA level was not affected by Mn2+ (Fig. 4B). Given that BosR is a Zn-binding protein, increased intracellular Mn2+ levels may also affect the function of BosR. Nevertheless, our study demonstrates that the major effect of Mn2+ on BosR is at the level of BosR protein, by affecting either the translation of BosR or the stability of BosR protein. One attractive hypothesis is that there is a yet-to-be-identified Mn2+-dependent protease that governs the turnover rate of the BosR protein. We are currently in the process of testing this possibility.
In summary, we have demonstrated that the two transition metals Mn2+ and Zn2+ play reciprocal roles in regulation of RpoS in B. burgdorferi by affecting the level of BosR at the posttranscriptional level. Whether the activity of RpoN or Rrp2 phosphorylation is also affected by Mn2+ or Zn2+ remains to be determined. Our data also led us to postulate that many environmental cues and host signals may collectively influence the Mn2+/Zn2+ ratio and subsequently modulate the BosR protein and rpoS transcription levels (Fig. 7). Given that human blood contains a high concentration of Zn2+ relative to a low concentration of Mn2+ (90–92), it is likely that the tick midgut during the feeding is under low Mn2+ and high Zn2+ conditions that favor the production of BosR and activation of the RpoS pathway. This model cannot explain the situation in the feeding larvae when spirochetes migrate from mammals to larval midguts, in which the RpoS pathway is not activated even in the presence of mammalian blood. The mechanism underlying the difference in gene regulation of B. burgdorferi between feeding nymphs and feeding larvae remains unclear but may be due to differences in temperature shift and growth rate changes that spirochetes encounter between the processes of acquisition (from mammals to larvae) and transmission (from nymphs to mammals) (4, 100). Nevertheless, this study demonstrates that Mn2+ is a global regulatory element in B. burgdorferi and supports the emerging notion that Mn2+ and Zn2+ play a critical role in regulation of virulence in bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge M. V. Norgard, Z. Ouyang, and P. Rosa for strains and plasmids. We thank W. P. Robarge and Kim Hutchison at North Carolina State University for ICP-MS analysis.
Funding for this work was partially provided by NIH grants AI083640 and AI085242, Indiana INGEN and METACyt grants of Indiana University, funded by the Lilly Endowment, Inc. (to X.F.Y.), and by the National Science Foundation of China (81171611) (to Y. L). Trainee B.T. was supported by NIH T32 AI060519. This investigation was partially conducted in a facility with support from research facilities improvement program grant number C06 RR015481-01 from the National Center for Research Resources, NIH.
Footnotes
Published ahead of print 20 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00507-13.
REFERENCES
- 1. Burgdorfer W, Barbour A, Hayes S, Benach J, Grunwaldt E, Davis J. 1982. Lyme disease—a tick-borne spirochetosis? Science 18:1317–1319 [DOI] [PubMed] [Google Scholar]
- 2. Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733–740 [DOI] [PubMed] [Google Scholar]
- 3. Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740–742 [DOI] [PubMed] [Google Scholar]
- 4. Schwan TG, Piesman J. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosa PA, Tilly K, Stewart PE. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3:129–143 [DOI] [PubMed] [Google Scholar]
- 6. Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65:479–499 [DOI] [PubMed] [Google Scholar]
- 7. Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Silva AM, Telford SR, III, Brunet LR, Barthold SW, Fikrig E. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montgomery RR, Malawista SE, Feen KJ, Bockenstedt LK. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 106:561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457–468 [DOI] [PubMed] [Google Scholar]
- 12. Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohnishi J, Piesman J, de Silva AM. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. U. S. A. 98:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, Fikrig E. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fingerle V, Goettner G, Gern L, Wilske B, Schulte-Spechtel U. 2007. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int. J. Med. Microbiol. 297:97–107 [DOI] [PubMed] [Google Scholar]
- 19. Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. 2005. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U. S. A. 102:5162–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, Samuels DS, Norgard MV. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187:4822–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. 2007. Evidence that RpoS (sigmaS) in Borrelia burgdorferi is controlled directly by RpoN (sigma54/sigmaN). J. Bacteriol. 189:2139–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ouyang Z, Blevins JS, Norgard MV. 2008. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154:2641–2658 [DOI] [PubMed] [Google Scholar]
- 25. Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lybecker MC, Samuels DS. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075–1089 [DOI] [PubMed] [Google Scholar]
- 27. Lybecker MC, Abel CA, Feig AL, Samuels DS. 2010. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 78:622–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boylan JA, Posey JE, Gherardini FC. 2003. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 100:11684–11689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katona LI, Tokarz R, Kuhlow CJ, Benach J, Benach JL. 2004. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186:6443–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hyde JA, Shaw DK, Smith Iii R, Trzeciakowski JP, Skare JT. 2009. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74:1344–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, Norgard MV. 2009. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74:1331–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samuels DS, Radolf JD. 2009. Who is the BosR around here anyway? Mol. Microbiol. 74:1295–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. 2010. Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect. Immun. 78:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seshu J, Boylan JA, Gherardini FC, Skare JT. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 72:1580–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boylan JA, Hummel CS, Benoit S, Garcia-Lara J, Treglown-Downey J, Crane EJ, III, Gherardini FC. 2006. Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response. Mol. Microbiol. 59:475–486 [DOI] [PubMed] [Google Scholar]
- 37. Buck M, Gallegos MT, Studholme DJ, Guo Y, Gralla JD. 2000. The bacterial enhancer-dependent sigma 54 (sigma N) transcription factor. J. Bacteriol. 182:4129–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wigneshweraraj S, Bose D, Burrows PC, Joly N, Schumacher J, Rappas M, Pape T, Zhang X, Stockley P, Severinov K, Buck M. 2008. Modus operandi of the bacterial RNA polymerase containing the σ54 promoter-specificity factor. Mol. Microbiol. 68:538–546 [DOI] [PubMed] [Google Scholar]
- 39. Hoerter JD, Arnold AA, Kuczynska DA, Shibuya A, Ward CS, Sauer MG, Gizachew A, Hotchkiss TM, Fleming TJ, Johnson S. 2005. Effects of sublethal UVA irradiation on activity levels of oxidative defense enzymes and protein oxidation in Escherichia coli. J. Photochem. Photobiol. B 81:171–180 [DOI] [PubMed] [Google Scholar]
- 40. Boughammoura A, Matzanke BF, Bottger L, Reverchon S, Lesuisse E, Expert D, Franza T. 2008. Differential role of ferritins in iron metabolism and virulence of the plant-pathogenic bacterium Erwinia chrysanthemi 3937. J. Bacteriol. 190:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouyang Z, Deka RK, Norgard MV. 2011. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 7:e1001272. 10.1371/journal.ppat.1001272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcia Vescovi E, Soncini FC, Groisman EA. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165–174 [DOI] [PubMed] [Google Scholar]
- 43. Ellermeier JR, Slauch JM. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, Hassan HM. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teixido L, Carrasco B, Alonso JC, Barbe J, Campoy S. 2011. Fur activates the expression of Salmonella enterica pathogenicity island 1 by directly interacting with the hilD operator in vivo and in vitro. PLoS One 6:e19711. 10.1371/journal.pone.0019711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. 2007. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109:353–358 [DOI] [PubMed] [Google Scholar]
- 47. Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, Dooley S, Hentze MW, Muckenthaler MU. 2010. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood 115:2657–2665 [DOI] [PubMed] [Google Scholar]
- 48. Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965 [DOI] [PubMed] [Google Scholar]
- 49. Ouyang Z, He M, Oman T, Yang XF, Norgard MV. 2009. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 106:3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Samuels DS. 1995. Electrotransformation of the Spirochete Borrelia burgdorferi, p 253–259 In Nickoloff JA. (ed), Methods in molecular biology. Humana Press, Totowa, NJ: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barbour AG. 1986. Cultivation of Borrelia: a historical overview. Zentralbl. Bakteriol. Mikrobiol. Hyg. Ser. A 263:11–14 [DOI] [PubMed] [Google Scholar]
- 53. Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521–525 [PMC free article] [PubMed] [Google Scholar]
- 54. Troxell B, Xu H, Yang XF. 2012. Borrelia burgdorferi, a pathogen that lacks iron, encodes a manganese-dependent superoxide dismutase essential for resistance to streptonigrin. J. Biol. Chem. 287:19284–19293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470–1479 [DOI] [PubMed] [Google Scholar]
- 57. Stevenson B, Schwan TG, Rosa PA. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang XF, Hubner A, Popova TG, Hagman KE, Norgard MV. 2003. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 71:5012–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gilbert MA, Morton EA, Bundle SF, Samuels DS. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63:1259–1273 [DOI] [PubMed] [Google Scholar]
- 60. Yang XF, Alani SM, Norgard MV. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, Schwartz I. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang P, Lutton A, Olesik J, Vali H, Li X. 2012. A novel iron- and copper-binding protein in the Lyme disease spirochaete. Mol. Microbiol. 86:1441–1451 [DOI] [PubMed] [Google Scholar]
- 63. Raju BV, Esteve-Gassent MD, Karna SL, Miller CL, Van Laar TA, Seshu J. 2011. Oligopeptide permease A5 modulates vertebrate host-specific adaptation of Borrelia burgdorferi. Infect. Immun. 79:3407–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. He M, Oman T, Xu H, Blevins J, Norgard MV, Yang XF. 2008. Abrogation of ospAB constitutively activates the Rrp2-RpoN-RpoS pathway (sigmaN-sigmaS cascade) in Borrelia burgdorferi. Mol. Microbiol. 70:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carroll JA, Garon CF, Schwan TG. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carpenter BM, Whitmire JM, Merrell DS. 2009. This is not your mother's repressor: the complex role of fur in pathogenesis. Infect. Immun. 77:2590–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hantke K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172–177 [DOI] [PubMed] [Google Scholar]
- 68. Aguirre JD, Clark HM, McIlvin M, Vazquez C, Palmere SL, Grab DJ, Seshu J, Hart PJ, Saito M, Culotta VC. 2013. A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288:8468–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72:844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Troxell B, Fink RC, Porwollik S, McClelland M, Hassan HM. 2011. The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 11:236. 10.1186/1471-2180-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492 [DOI] [PubMed] [Google Scholar]
- 72. Lindsay JA, Foster SJ. 2001. zur: a Zn(2+)-responsive regulatory element of Staphylococcus aureus. Microbiology 147:1259–1266 [DOI] [PubMed] [Google Scholar]
- 73. Patzer SI, Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321–24332 [DOI] [PubMed] [Google Scholar]
- 74. Dalet K, Gouin E, Cenatiempo Y, Cossart P, Hechard Y. 1999. Characterisation of a new operon encoding a Zur-like protein and an associated ABC zinc permease in Listeria monocytogenes. FEMS Microbiol. Lett. 174:111–116 [DOI] [PubMed] [Google Scholar]
- 75. Gaballa A, Helmann JD. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patzer SI, Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- 77. Brett PJ, Burtnick MN, Fenno JC, Gherardini FC. 2008. Treponema denticola TroR is a manganese- and iron-dependent transcriptional repressor. Mol. Microbiol. 70:396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Posey JE, Hardham JM, Norris SJ, Gherardini FC. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. U. S. A. 96:10887–10892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hazlett KR, Rusnak F, Kehres DG, Bearden SW, La Vake CJ, La Vake ME, Maguire ME, Perry RD, Radolf JD. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J. Biol. Chem. 278:20687–20694 [DOI] [PubMed] [Google Scholar]
- 80. Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gherardini F, Wolfe AJ, Yang XF. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 6:e1001104. 10.1371/journal.ppat.1001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Faulkner MJ, Hellmann JD. 2011. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid. Redox Signal. 15:175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tokarz R, Anderton JM, Katona LI, Benach JL. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hyde JA, Trzeciakowski JP, Skare JT. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Van Laar TA, Lin Y-H, Miller CL, Karna SLR, Chambers JP, Seshu J. 2012. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PLoS One 7:e38171. 10.1371/journal.pone.0038171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sze CW, Li C. 2011. Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi. Infect. Immun. 79:1270–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karna SL, Sanjuan E, Esteve-Gassent MD, Miller CL, Maruskova M, Seshu J. 2011. CsrA modulates levels of lipoproteins and key regulators of gene expression critical for pathogenic mechanisms of Borrelia burgdorferi. Infect. Immun. 79:732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wolfe AJ. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pruss BM, Wolfe AJ. 1994. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 12:973–984 [DOI] [PubMed] [Google Scholar]
- 89. Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JC. 2012. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 194:686–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vallee BL, Gibson JG., II 1948. The zinc content of normal human whole blood, plasma, leucocytes, and erythrocytes. J. Biol. Chem. 176:445–457 [PubMed] [Google Scholar]
- 91. Dawson JB, Walker BE. 1969. Direct determination of zinc in whole blood, plasma and urine by atomic absorption spectroscopy. Clin. Chim. Acta 26:465–475 [DOI] [PubMed] [Google Scholar]
- 92. Xu B, Chia SE, Ong CN. 1994. Concentrations of cadmium, lead, selenium, and zinc in human blood and seminal plasma. Biol. Trace Elem. Res. 40:49–57 [DOI] [PubMed] [Google Scholar]
- 93. Ikeda JS, Janakiraman A, Kehres DG, Maguire ME, Slauch JM. 2005. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J. Bacteriol. 187:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Posey JE, Gherardini FC. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651–1653 [DOI] [PubMed] [Google Scholar]
- 95. Bourret TJ, Boylan JA, Lawrence KA, Gherardini FC. 2011. Nitrosative damage to free and zinc-bound cysteine thiols underlies nitric oxide toxicity in wild-type Borrelia burgdorferi. Mol. Microbiol. 81:259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nguyen KT, Wu JC, Boylan JA, Gherardini FC, Pei D. 2007. Zinc is the metal cofactor of Borrelia burgdorferi peptide deformylase. Arch. Biochem. Biophys. 468:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Duckworth HW, Barber BH, Sanwal BD. 1973. The interaction of phosphoglucomutase with nucleotide inhibitors. J. Biol. Chem. 248:1431–1435 [PubMed] [Google Scholar]
- 98. Jedrzejas MJ, Chander M, Setlow P, Krishnasamy G. 2000. Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. EMBO J. 19:1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. 2007. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65:277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jutras BL, Chenail AM, Stevenson B. 2013. Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins. J. Bacteriol. 195:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


