Abstract
Antimicrobial catheter lock therapy is practiced to prevent lumenal-sourced infections of central venous catheters. Citrate has been used clinically as an anticoagulant in heparin-free catheter locks. Ethanol has also been widely studied as an antimicrobial lock solution component. This study reports on the synergy of glyceryl trinitrate (GTN) with citrate and ethanol in rapidly eradicating methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, Pseudomonas aeruginosa, and Candida albicans biofilms in an in vitro model for catheter biofilm colonization. GTN has a long history of intravenous use as a hypotensive agent. It is potentially attractive as a component of a catheter lock solution because its physiologic half-life is quite short and its metabolic pathways are known. A lock containing 7% citrate and 20% ethanol required 0.01% GTN to fully eradicate biofilms of all test organisms within 2 h in the model. This GTN concentration is below the levels where clinically significant hypotensive effects are expected.
INTRODUCTION
Central line-associated bloodstream infections (CLABSIs) have declined over the past decade but remain a significant medical problem, with a substantial cost burden to the health care system. There are an estimated 250,000 CLABSIs in the United States (1), with an estimated treatment cost of $45,000 or more per infection (2). Significant portions of CLABSIs annually are associated with hemodialysis treatment (3) and treatment of critically ill patients. CLABSIs that present after the first week of catheterization tend to be associated with colonization of the catheter lumenal surfaces (4, 5) and biofilm formation on those surfaces. Colonizing organisms organize within hours into biofilm structures which can serve as a source of CLABSI and can be highly resistant to eradication by antibiotic treatments. Approaches to reducing lumenal colonization include rigorous provider hygiene and use of antimicrobial swabs when connectors are manipulated. Even with these precautions, much of the lumenal surfaces remain vulnerable to colonization. Another approach to combatting lumenal infections has been the use of antimicrobial lock therapy (ALT). Catheter lumens are hydraulically locked when not in use. Typically, lock solutions include anticoagulants to inhibit the formation of blood clots, which can occlude flow through catheters. Heparin has traditionally been used as an anticoagulant; however, there have been concerns about its purity (6), as well as its potential to promote bacterial biofilm colonization (7). Antimicrobial locks have generally fallen into the categories of antibiotic locks and nonantibiotic, antiseptic locks. Antibiotic locks have been pursued both for catheter salvage and for infection prevention. Antibiotic catheter locks have recently been reviewed (8). Significant issues limiting the use of antibiotic locks for prevention are their higher costs and potential to promote the development of antibiotic resistance. We report here on the utility of glyceryl trinitrate (GTN) compositions as nonantibiotic locks. GTN was studied because it is used clinically in intravenous infusions through catheters to control hypertension, has a short half-life, and has been reported to have antimicrobial activity against planktonic organisms (9).
MATERIALS AND METHODS
In vitro assessment of GTN for lock application employed the modified Kuhn's method (10) to test for eradication of biofilm within 2 h of lock exposure on low-surface-energy silicone rubber surfaces. Silicone was utilized since lower-surface-energy materials tend to hinder wetting by aqueous-based locks and present a worst case for an intervention of limited duration to eradicate biofilm. Two hours was selected as the exposure duration since, in our experience, that is the longest interval that vascular access can be removed from critically ill patients for ALT (longer ALT dwell times are possible for non-critically ill patients; however, a lock that is effective within 2 h would also be effective with longer dwell times). Briefly, silicone discs (1-cm diameter) were placed into a 24-well tissue culture plate and incubated overnight at 37°C with 1 ml human plasma. The plasma was removed and replaced with 1 ml of a 5.5 × 105 CFU/ml inoculum of the challenge organism in Mueller-Hinton broth II (MHB). The plates were incubated for 24 h at 37°C. The inoculum was then removed, and discs were washed by shaking for 30 min in 0.9% sterile saline to remove nonadherent organisms. After washing, discs were placed in 1 ml of lock solution and incubated at 37°C for 2 h. The discs were then removed and placed in 5 ml of 0.9% sterile saline and sonicated (60 Hz and 150 W) for 15 min to disrupt any remaining biofilm. The resulting solution was then quantitatively cultured by making serial dilutions in 0.9% sterile saline and plating on agar plates (Trypticase soy agar [TSA] plus 5% sheep blood for all bacterial organisms and Sabouraud dextrose agar for yeasts). The challenge organisms were clinical isolates representative of key Gram-positive, Gram-negative, and fungal BSI pathogens (methicillin-resistant Staphylococcus aureus [MRSA], methicillin-resistant Staphylococcus epidermidis [MRSE], multidrug-resistant Pseudomonas aeruginosa, and Candida albicans).
GTN for intravenous use is supplied as a 5-mg/ml concentrate which also contains 30% ethanol (EtOH) and 30% propylene glycol (PG) to facilitate dissolution in aqueous diluents used for infusion. Thus, aqueous dilutions of intravenous GTN concentrate also contain ethanol and propylene glycol at 60 times the final concentration of GTN. Locks prepared from GTN concentrate (American Regent, Shirley, NY) were diluted in aqueous trisodium citrate dihydrate (citrate) solution to a final citrate concentration of 7%. A first set of experiments were performed using GTN concentrate plus citrate solutions. Ethanol and propylene glycol carried over from diluting the GTN concentrate and were considered in calculating the final concentrations. A second set of experiments was performed where anhydrous ethanol was added to increase the ethanol concentrations to up to 20% while at the same time progressively decreasing the GTN concentration (0.05% to 0.005%) in order to determine the minimum concentration of GTN required to eradicate biofilms of all challenge organisms at a fixed ethanol concentration. In both sets of experiments, the ratio of propylene glycol to GTN in the concentrate (60:1) was preserved in the dilutions. The compositions of the solutions and controls tested in this series of experiments are tabulated in Table 1. Controls for these experiments (without GTN) included citrate (Cit) alone, ethanol (EtOH) alone, propylene glycol (PG) alone, ethanol-propylene glycol combinations, and citrate-ethanol-propylene glycol combinations.
Table 1.
Summary of lock compositions tested in citrate-glyceryl trinitrate concentrate-ethanol experiments
| Component(s)a | % of component |
Results in figure(s)b: | |||
|---|---|---|---|---|---|
| GTN (wt/vol) | EtOH (vol/vol) | PG (vol/vol) | Cit (wt/vol) | ||
| MHB | 1, 2, 3 | ||||
| PG | 0 | 0 | 6 | 0 | 1 |
| PG | 0 | 0 | 9 | 0 | 1 |
| EtOH | 0 | 6 | 0 | 0 | 1 |
| EtOH | 0 | 9 | 0 | 0 | 1 |
| EtOH-PG | 0 | 6 | 6 | 0 | 1 |
| EtOH-PG | 0 | 9 | 9 | 0 | 1, 2A |
| Cit | 0 | 0 | 0 | 7 | 1 |
| EtOH-PG-Cit | 0 | 6 | 6 | 7 | 1, 2B |
| EtOH-PG-Cit | 0 | 9 | 9 | 7 | 1 |
| GTN-EtOH-PG | 0.10 | 6 | 6 | 0 | 2A |
| GTN-EtOH-PG | 0.15 | 9 | 9 | 0 | 2A |
| GTN-EtOH-PG-Cit | 0.10 | 6 | 6 | 7 | 2B |
| GTN-EtOH-PG-Cit | 0.05 | 10 | 3 | 7 | 3A |
| GTN-EtOH-PG-Cit | 0.02 | 10 | 1.2 | 7 | 3A |
| EtOH-PG-Cit | 0 | 10 | 3 | 7 | 3A |
| GTN-EtOH-PG-Cit | 0.02 | 15 | 1.2 | 7 | 3B |
| GTN-EtOH-PG-Cit | 0.01 | 15 | 0.6 | 7 | 3B |
| EtOH-PG-Cit | 0 | 15 | 1.2 | 7 | 3B |
| GTN-EtOH-PG-Cit | 0.01 | 20 | 0.6 | 7 | 3C |
| GTN-EtOH-PG-Cit | 0.005 | 20 | 0.3 | 7 | 3C |
| EtOH-PG-Cit | 0 | 20 | 0.6 | 7 | 3C |
GTN, glyceryl trinitrate; EtOH, ethanol; PG, propylene glycol; Cit, trisodium citrate; MHB, Mueller Hinton broth II. MHB was used as a positive control for each experiment.
Figure(s) where biofilm eradication data are presented for the particular composition.
RESULTS
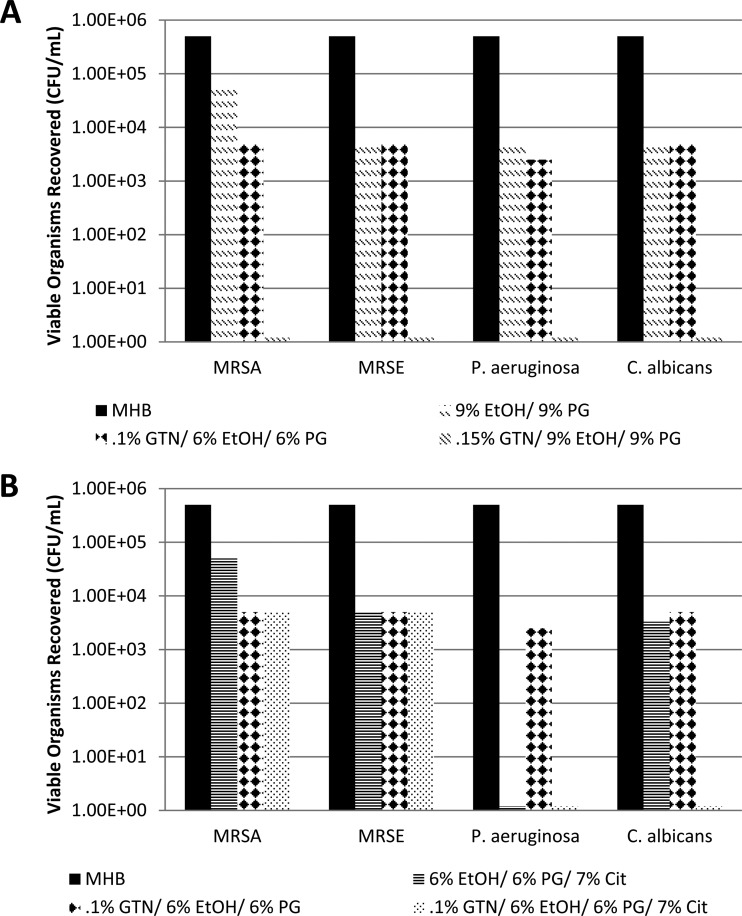
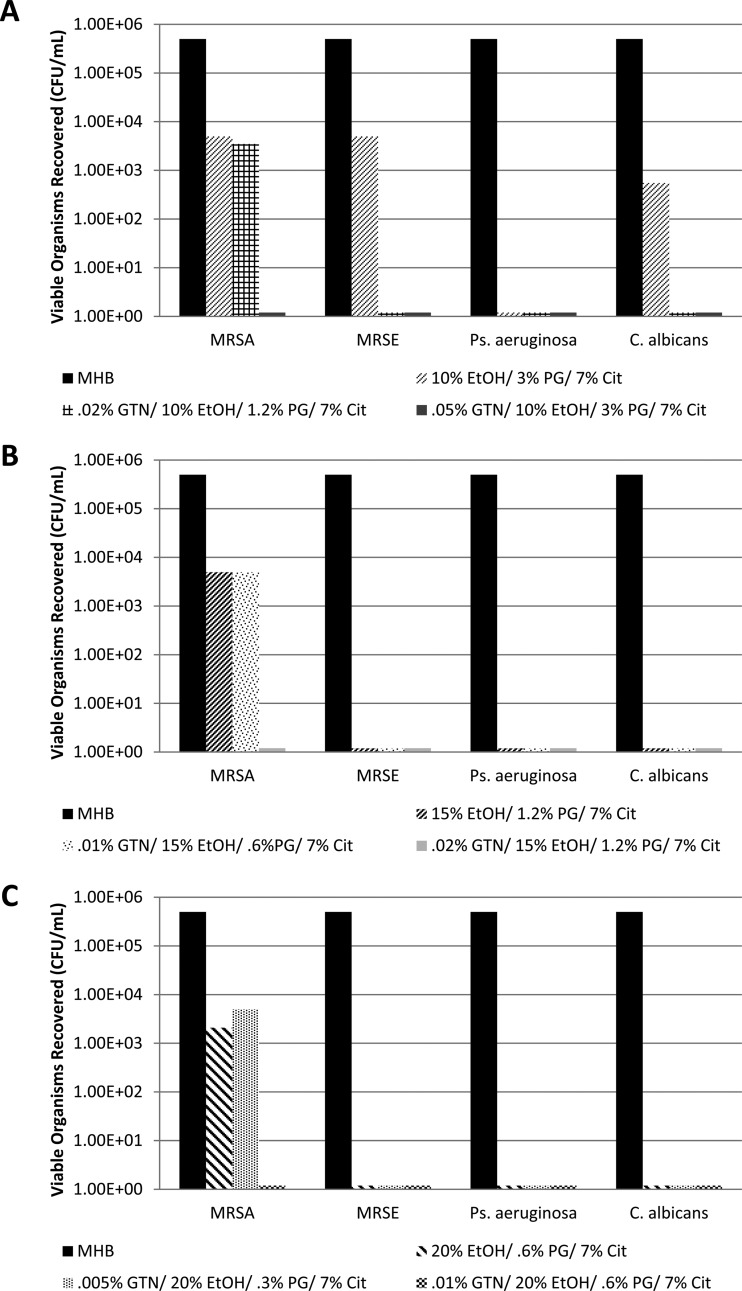
Recoveries from the silicone disks for controls without GTN are presented in Figure 1A to D. Due to the number of components in the GTN concentrate, the controls encompassed all combinations of ethanol, propylene glycol, and citrate at the same concentrations as in the GTN-containing locks in order to isolate the contribution of GTN. All control combinations were tested separately for each test organism. Recoveries for GTN-EtOH-PG and GTN-EtOH-PG-Cit locks are presented in Figure 2A (GTN-EtOH-PG concentrations required for complete eradication of all biofilms) and B (GTN-EtOH-PG-Cit concentrations required to eradicate Pseudomonas aeruginosa and Candida albicans biofilms). Recoveries from the silicone disks for GTN-EtOH-PG-Cit locks supplemented with additional ethanol are presented in Figure 3A (10% final ethanol), 3B (15% final ethanol), and 3C (20% final ethanol); for each target final ethanol concentration, the GTN concentration required for full eradication of all biofilms was titrated at fixed ethanol and citrate concentrations.
Fig 1.
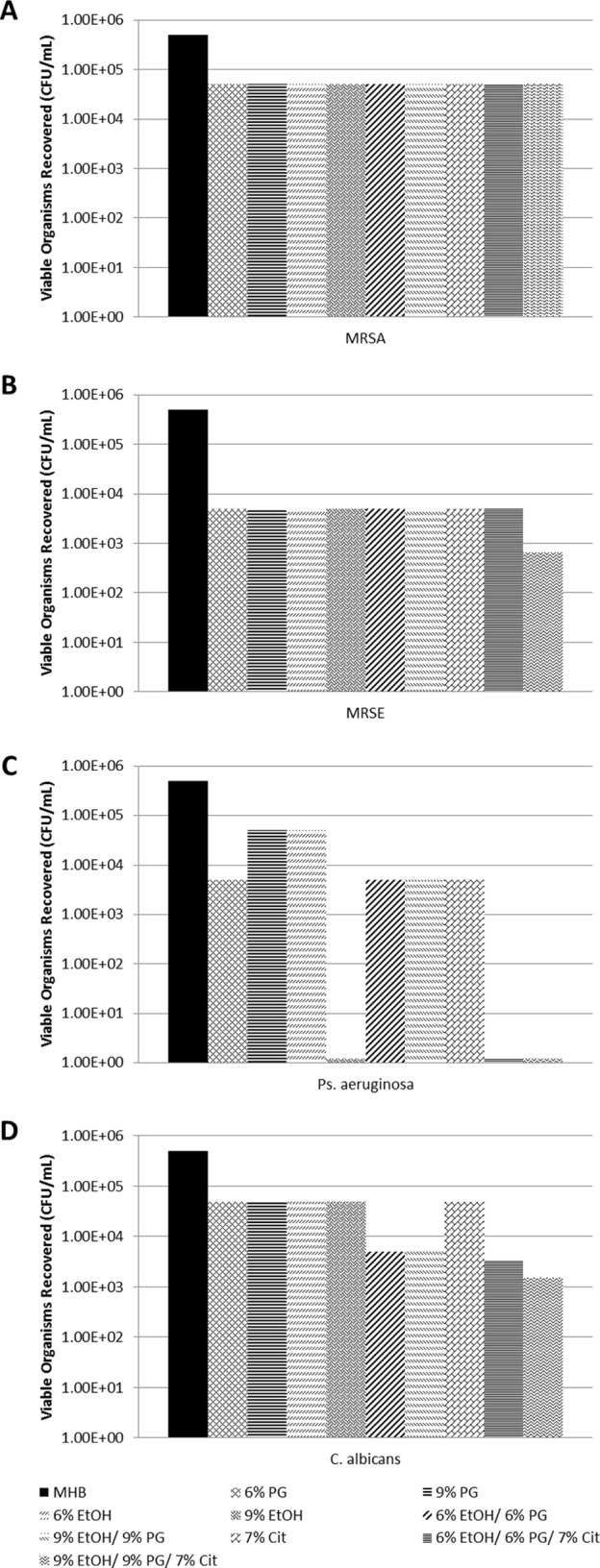
Effect of control locks (not containing GTN) on biofilms of MRSA (A), MRSE (B), P. aeruginosa (C), and C. albicans (D). Controls were Mueller-Hinton broth II (MHB), 6% ethanol (EtOH), 9% EtOH, 6% propylene glycol (PG), 9% PG, 7% citrate (Cit), 6% EtOH–6% PG, 9% EtOH–9% PG, 6% /EtOH–6% PG–7% Cit, and 9% EtOH–9% PG–7% Cit.
Fig 2.
(A) GTN concentration of 0.15% in diluted GTN concentrate is required for biofilm eradication of all organisms. Relevant controls to isolate the contribution of GTN are presented in Figure 1. (B) Synergy is observed between 7% citrate and 0.1% GTN for eradication of C. albicans biofilms.
Fig 3.
Effect on biofilm eradication of dilution of GTN concentrate at a constant citrate concentration of 7% and supplementation with additional ethanol at the concentration indicated in each panel.
Dilution of the GTN concentrate to 0.1% GTN (with 6% EtOH and 6% PG carried over from the concentrate) was unable to fully eradicate the biofilm of any challenge organism within 2 h (Fig. 2A). 0.15% GTN (with 9% EtOH and 9% PG carried over) was required to fully eradicate biofilms of all tested organisms within 2 h. Furthermore, 7% citrate alone and 9% EtOH plus 9% PG without GTN (or 6% EtOH plus 6% PG without GTN) were unable to fully eradicate biofilm within 2 h for any of the organisms. Interestingly, 9% ethanol alone (no PG) was able to fully eradicate P. aeruginosa biofilm. The 7% citrate plus 6% EtOH plus 6% PG control was able to eradicate P. aeruginosa biofilm but not the other biofilms. The addition of 7% citrate to 0.1% GTN plus 6% EtOH plus 6% PG was able to fully eradicate biofilm for C. albicans, showing synergy for this organism (Fig. 2B). It was not able to fully eradicate MRSA or MRSE biofilm within 2 h. Both the 0.1% and 0.15% concentrations of GTN could be sufficiently high to potentially cause hypotension if inadvertently flushed.
The results in Figure 3 show that the concentration of GTN required for complete biofilm eradication of all organisms within 2 h could be reduced to minimally hypotensive levels by adding both citrate and additional ethanol in dilutions of GTN concentrate. While holding 7% citrate constant and with 10% ethanol, the concentration of GTN required for full eradication of all organisms was reduced to 0.05% (Fig. 3A). At 15% ethanol (and 7% citrate), the concentration of GTN required for complete eradication was further reduced to 0.02% (Fig. 3B), and at 20% ethanol (and 7% citrate), GTN was fully effective at 0.01% (Fig. 3C). Of all of the organisms tested, MRSA biofilms were the most resistant to eradication by this combination. For MRSA, the synergistic reductions in viable biofilm organisms by the GTN-EtOH-PG-Cit combinations were significant relative to the reductions by the same concentrations of EtOH-PG-Cit alone (P = 0.047).
DISCUSSION
This is the first study, to our knowledge, to demonstrate the synergistic activity of GTN and citrate in rapidly eradicating biofilms associated with CLABSI. P. aeruginosa biofilms were most susceptible to the combination of agents, followed by C. albicans, MRSE, and MRSA. The concentration of GTN required for the eradication of all CLABSI pathogens tested could be reduced to clinically acceptable levels (0.01%) by further supplementing with additional ethanol. The 20% ethanol concentration required at this GTN concentration is well below the ethanol concentrations employed in previously studied ethanol locks.
Citrate has been a popular chelator for use in ALT since it provides both antimicrobial and anticoagulant activity. Citrate concentrations of 4 to 7% have been used for anticoagulant effect; however, concentrations as high as 30 to 46% are needed for antimicrobial effect. Safety questions have been raised for the antimicrobial concentrations of citrate (11), and degradation of heart function has been reported with 10% citrate locks (12). Anticoagulant levels (less than 10%) can safely contact blood (13). Other antimicrobial agents have been added to supplement anticoagulant levels of citrate. One formulation subjected to a large clinical trial with a 48-h lock duration contained 7% citrate, methylene blue, and methyl and propyl parabens. While trending toward fewer infections, the reduction in catheter infections from methylene blue-paraben-citrate lock over the number with heparin was not significant (14). Fewer catheter occlusion complications or other adverse reactions were reported for the 7% citrate lock composition than for the heparin lock control in this study. Another trial reported a significant reduction in infections in immunocompromised pediatric patients with a 1.35% taurolidine–4% citrate lock versus the number with heparin; however, 20% of the taurolidine-citrate group reported side effects (15).
Glyceryl trinitrate (GTN) has a long history of intravenous use in treating hypertension. Recently, its value in wound healing and as an antimicrobial agent has been appreciated (9, 16). From a safety standpoint, the worst-case systemic exposure occurs if a lock is inadvertently flushed, in which case a bolus dose is presented. Bolus GTN has been studied in numerous clinical trials over a range of doses from 100 μg to 2 mg in applications ranging from emergency medicine to complicated child birth (17–20). GTN has a very short half-life (1 to 3 min), so any systemic effects are transient. Bolus doses of 200 μg of GTN have been well tolerated and had no clinically significant adverse effects. Studies have shown that these transient effects are usually brief, non-clinically relevant, hypotensive episodes of approximately 10 mm Hg or less. Additionally, several of the clinical studies allowed multiple doses of GTN to be administered sequentially without issue. This includes effects on both fetus and mother when used for uterine relaxation during complex childbirth.
Ethanol has also been well studied as a lock component. In a clinical trial, 15 min of locking with 70% ethanol followed by an intravenous flush gave a 10-fold higher incidence of complaints than heparin lock (21). The complaints were typically transient adverse reactions associated with inebriation. These side effects were not reported in a different pilot clinical trial using 70% ethanol to salvage central venous catheters (CVCs) in patients experiencing bacteremias (22), as well as in a meta-analysis of 70% ethanol lock clinical trials (23). Sixty percent ethanol was not able to completely eradicate Staphylococcal biofilms in vitro within 20 min (24), and in a clinical trial, a 1- to 3-h lock with 50% ethanol did not show a significant reduction in infection (25). The combination of 30% ethanol and 4% citrate has been shown to eradicate planktonic organisms in vitro (26); however, its performance against biofilm requires further study. It has even been reported that ethanol treatment can enhance biofilm formation for Staphylococcus aureus biofilms (27).
Chelators, such as citrate, are known to interfere with the exopolysaccharide glycocalyx formed by biofilms and enhance the permeability of other antimicrobial agents within the extracellular matrix of biofilms (28). GTN is known to be metabolized to nitric oxide by nitrate reductase enzymes (29). While not assessed in this study, it is possible that the mechanism of synergy seen here is due to the combined effects of nitric oxide production from GTN and biofilm disruption by the chelator-nitric oxide combination. Staphylococci are known to be able to oxidize nitric oxide, which detoxifies it (30). Hence, the greater GTN concentrations required to fully eradicate MRSA and MRSE biofilms may reflect the intrinsic nitric oxide detoxification pathways of these bacteria.
The 0.01% GTN–20% EtOH–0.6% PG–7% Cit lock presents a 33% reduction in the concentration of ethanol from a 30% ethanol–4% citrate lock (26) and 20% less ethanol than a minocycline-EDTA–25% ethanol lock (which was also able to fully eradicate biofilm within 2 h) (31). The reduced ethanol concentration in the GTN lock presents a greater margin of safety from adverse clinical reactions (21), as well as a greater margin of safety from effects on catheter mechanical strength (32). In conclusion, the GTN lock composition reported here merits further in vivo and clinical study to establish its utility in preventing catheter infection or in salvaging infected catheters, based on its reduced ethanol levels, absence of antibiotics, and safe citrate and GTN concentrations. Since some plastics are known to partially absorb GTN (33), the effect of contact with catheters on the potency of the lock needs further study. Also, the GTN lock composition studied here requires additional testing for its effect on catheter mechanical properties.
ACKNOWLEDGMENTS
This work was not supported by a grant. All authors have no conflicts to disclose.
Footnotes
Published ahead of print 13 May 2013
REFERENCES
- 1. O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. 2011. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 52:e162–e193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. 2006. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit. Care Med. 34:2084–2089 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2011. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb. Mortal. Wkly. Rep. 60:243–248 [PubMed] [Google Scholar]
- 4. Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168:400–407 [DOI] [PubMed] [Google Scholar]
- 5. Safdar N, Maki DG. 2004. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med. 30:62–67 [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. 2008. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 358:2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanks RM, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596–4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snaterse M, Ruger W, Scholte Op Reimer WJ, Lucas C. 2010. Antibiotic-based catheter lock solutions for prevention of catheter-related bloodstream infection: a systematic review of randomised controlled trials. J. Hosp. Infect. 75:1–11 [DOI] [PubMed] [Google Scholar]
- 9. Palmeira-de-Oliveira A, Ramos AR, Gaspar C, Palmeira-de-Oliveira R, Gouveia P, Martinez-de-Oliveira J. 2012. In vitro anti-Candida activity of lidocaine and nitroglycerin: alone and combined. Infect. Dis. Obstet. Gynecol. 2012:727248. 10.1155/2012/727248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polaschegg HD, Sodemann K. 2003. Risks related to catheter locking solutions containing concentrated citrate. Nephrol. Dial. Transplant. 18:2688–2690 [DOI] [PubMed] [Google Scholar]
- 12. Bagnall-Reeb H. 2004. Evidence for the use of antibiotic lock technique. J. Infus. Nurs. 27:118–122 [DOI] [PubMed] [Google Scholar]
- 13. Schilcher G, Scharnagl H, Horina JH, Ribitsch W, Rosenkranz AR, Stojakovic T, Polaschegg HD. 2012. Trisodium citrate induced protein precipitation in haemodialysis catheters might cause pulmonary embolism. Nephrol. Dial. Transplant. 27:2953–2957 [DOI] [PubMed] [Google Scholar]
- 14. Maki DG, Ash SR, Winger RK, Lavin P. 2011. A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: a multi-center, controlled, randomized trial. Crit. Care Med. 39:613–620 [DOI] [PubMed] [Google Scholar]
- 15. Dumichen MJ, Seeger K, Lode HN, Kuhl JS, Ebell W, Degenhardt P, Singer M, Geffers C, Querfeld U. 2012. Randomized controlled trial of taurolidine citrate versus heparin as catheter lock solution in paediatric patients with haematologic malignancies. J. Hosp. Infect. 80:304–309 [DOI] [PubMed] [Google Scholar]
- 16. Gorfine SR. 1995. Treatment of benign anal disease with topical nitroglycerin. Dis. Colon Rectum 38:453–457 [DOI] [PubMed] [Google Scholar]
- 17. Hill NS, Antman EM, Green LH, Alpert JS. 1981. Intravenous nitroglycerin. A review of pharmacology, indications, therapeutic effects and complications. Chest 79:69–76 [DOI] [PubMed] [Google Scholar]
- 18. Pullen KM, Riley ET, Waller SA, Taylor L, Caughey AB, Druzin ML, El-Sayed YY. 2007. Randomized comparison of intravenous terbutaline vs nitroglycerin for acute intrapartum fetal resuscitation. Am. J. Obstet. Gynecol. 197:414.e1-414.e6 [DOI] [PubMed] [Google Scholar]
- 19. Zalenski RJ, Levy P, Compton S, Delgado G, Welch R, Waselewsky D. 2004. The feasibility of treating severe acute congestive heart failure with bolus intravenous nitroglycerin. Ann. Emerg. Med. 44:S134–S135 [Google Scholar]
- 20. Levy P, Compton S, Welch R, Delgado G, Jennett A, Penugonda N, Dunne R, Zalenski R. 2007. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann. Emerg. Med. 50:144–152 [DOI] [PubMed] [Google Scholar]
- 21. Slobbe L, Doorduijn JK, Lugtenburg PJ, El Barzouhi A, Boersma E, van Leeuwen WB, Rijnders BJ. 2010. Prevention of catheter-related bacteremia with a daily ethanol lock in patients with tunnelled catheters: a randomized, placebo-controlled trial. PLoS One 5:e10840. 10.1371/journal.pone.0010840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broom J, Woods M, Allworth A, McCarthy J, Faoagali J, Macdonald S, Pithie A. 2008. Ethanol lock therapy to treat tunneled central venous catheter-associated blood stream infections: results from a prospective trial. Scand. J. Infect. Dis. 40:399–406 [DOI] [PubMed] [Google Scholar]
- 23. Oliveira C, Nasr A, Brindle M, Wales PW. 2012. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: a meta-analysis. Pediatrics 129:318–329 [DOI] [PubMed] [Google Scholar]
- 24. Balestrino D, Souweine B, Charbonnel N, Lautrette A, Aumeran C, Traore O, Forestier C. 2009. Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol. Dial. Transplant. 24:3204–3209 [DOI] [PubMed] [Google Scholar]
- 25. Crnich CJ, Duster M, Jones A, Maki DG. 2009. Prospective randomized double-blind trial of an ethanol lock for prevention of CLABSI, poster K-1234. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 26. Takla TA, Zelenitsky SA, Vercaigne LM. 2008. Effectiveness of a 30% ethanol/4% trisodium citrate locking solution in preventing biofilm formation by organisms causing haemodialysis catheter-related infections. J. Antimicrob. Chemother. 62:1024–1026 [DOI] [PubMed] [Google Scholar]
- 27. Redelman CV, Maduakolam C, Anderson GG. 2012. Alcohol treatment enhances Staphylococcus aureus biofilm development. FEMS Immunol. Med. Microbiol. 66:411–418 [DOI] [PubMed] [Google Scholar]
- 28. Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. 2008. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr. Opin. Infect. Dis. 21:385–392 [DOI] [PubMed] [Google Scholar]
- 29. Blehert DS, Knoke KL, Fox BG, Chambliss GH. 1997. Regioselectivity of nitroglycerin denitration by flavoprotein nitroester reductases purified from two Pseudomonas species. J. Bacteriol. 179:6912–6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richardson AR, Dunman PM, Fang FC. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol. Microbiol. 61:927–939 [DOI] [PubMed] [Google Scholar]
- 31. Maiefski M, Rupp ME, Hermsen ED. 2009. Ethanol lock technique: review of the literature. Infect. Control Hosp. Epidemiol. 30:1096–1108 [DOI] [PubMed] [Google Scholar]
- 32. Vercaigne LM, Takla TA, Raghavan J. 2010. Long-term effect of an ethanol/sodium citrate locking solution on the mechanical properties of hemodialysis catheters. J. Vasc. Access 11:12–16 [DOI] [PubMed] [Google Scholar]
- 33. Treleano A, Wolz G, Brandsch R, Welle F. 2009. Investigation into the sorption of nitroglycerin and diazepam into PVC tubes and alternative tube materials during application. Int. J. Pharm. 369:30–37 [DOI] [PubMed] [Google Scholar]